Mina Mousa
Integrative Imaging Informatics for Cancer Research: Workflow Automation for Neuro-oncology (I3CR-WANO)
Oct 06, 2022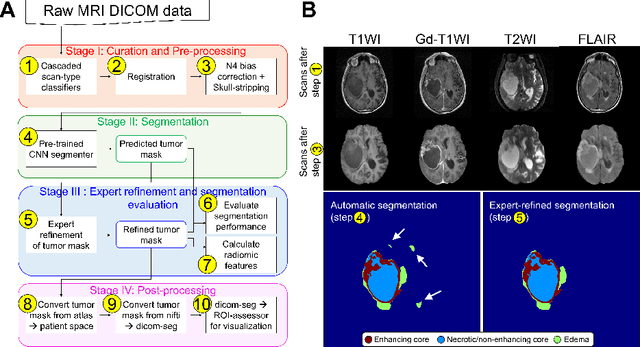
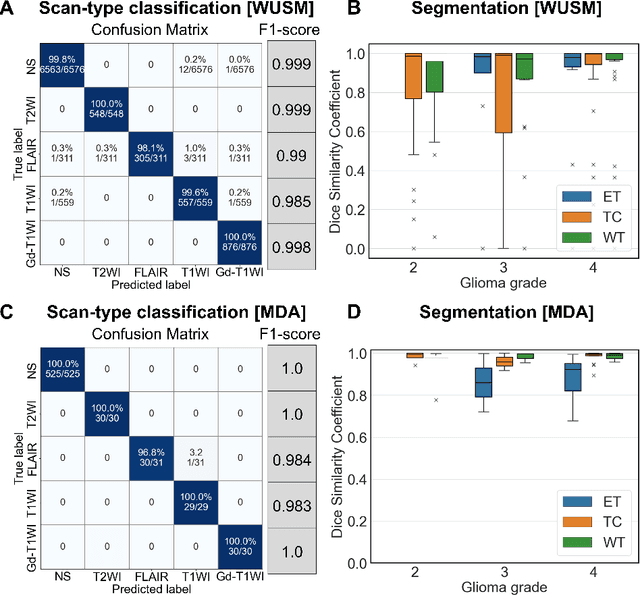
Abstract:Efforts to utilize growing volumes of clinical imaging data to generate tumor evaluations continue to require significant manual data wrangling owing to the data heterogeneity. Here, we propose an artificial intelligence-based solution for the aggregation and processing of multisequence neuro-oncology MRI data to extract quantitative tumor measurements. Our end-to-end framework i) classifies MRI sequences using an ensemble classifier, ii) preprocesses the data in a reproducible manner, iii) delineates tumor tissue subtypes using convolutional neural networks, and iv) extracts diverse radiomic features. Moreover, it is robust to missing sequences and adopts an expert-in-the-loop approach, where the segmentation results may be manually refined by radiologists. Following the implementation of the framework in Docker containers, it was applied to two retrospective glioma datasets collected from the Washington University School of Medicine (WUSM; n = 384) and the M.D. Anderson Cancer Center (MDA; n = 30) comprising preoperative MRI scans from patients with pathologically confirmed gliomas. The scan-type classifier yielded an accuracy of over 99%, correctly identifying sequences from 380/384 and 30/30 sessions from the WUSM and MDA datasets, respectively. Segmentation performance was quantified using the Dice Similarity Coefficient between the predicted and expert-refined tumor masks. Mean Dice scores were 0.882 ($\pm$0.244) and 0.977 ($\pm$0.04) for whole tumor segmentation for WUSM and MDA, respectively. This streamlined framework automatically curated, processed, and segmented raw MRI data of patients with varying grades of gliomas, enabling the curation of large-scale neuro-oncology datasets and demonstrating a high potential for integration as an assistive tool in clinical practice.
The Brain Tumor Sequence Registration Challenge: Establishing Correspondence between Pre-Operative and Follow-up MRI scans of diffuse glioma patients
Dec 13, 2021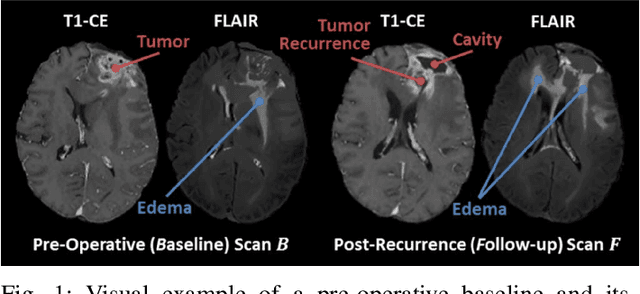
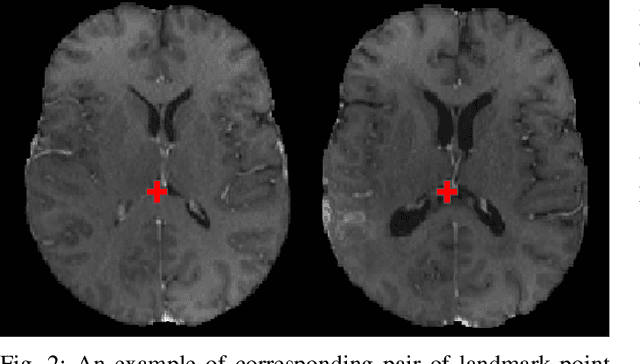
Abstract:Registration of longitudinal brain Magnetic Resonance Imaging (MRI) scans containing pathologies is challenging due to tissue appearance changes, and still an unsolved problem. This paper describes the first Brain Tumor Sequence Registration (BraTS-Reg) challenge, focusing on estimating correspondences between pre-operative and follow-up scans of the same patient diagnosed with a brain diffuse glioma. The BraTS-Reg challenge intends to establish a public benchmark environment for deformable registration algorithms. The associated dataset comprises de-identified multi-institutional multi-parametric MRI (mpMRI) data, curated for each scan's size and resolution, according to a common anatomical template. Clinical experts have generated extensive annotations of landmarks points within the scans, descriptive of distinct anatomical locations across the temporal domain. The training data along with these ground truth annotations will be released to participants to design and develop their registration algorithms, whereas the annotations for the validation and the testing data will be withheld by the organizers and used to evaluate the containerized algorithms of the participants. Each submitted algorithm will be quantitatively evaluated using several metrics, such as the Median Absolute Error (MAE), Robustness, and the Jacobian determinant.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge