John Wood
StyleGAN2-based Out-of-Distribution Detection for Medical Imaging
Jul 10, 2023
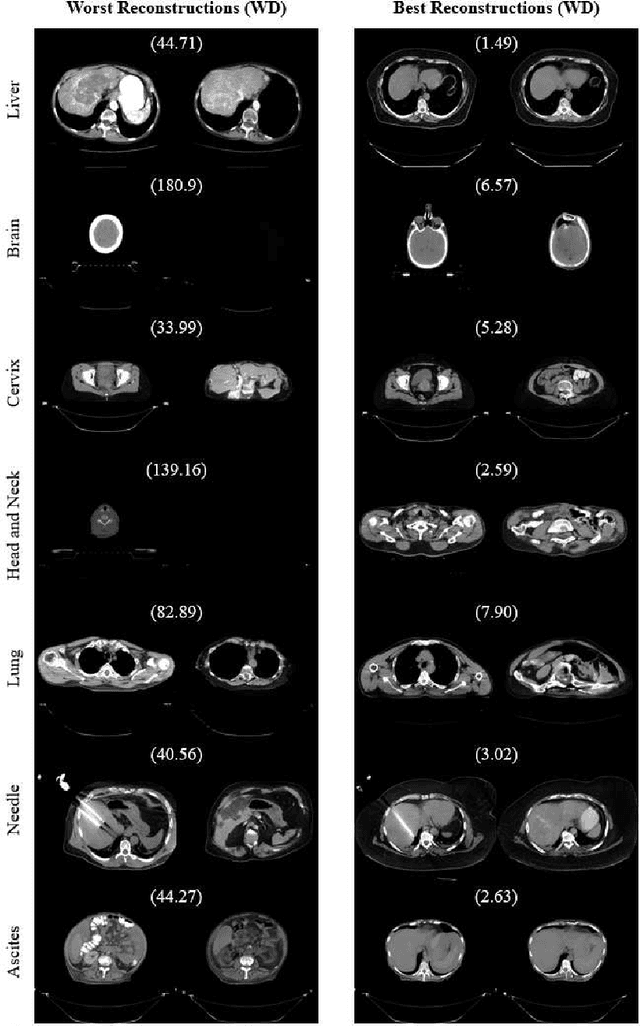
Abstract:One barrier to the clinical deployment of deep learning-based models is the presence of images at runtime that lie far outside the training distribution of a given model. We aim to detect these out-of-distribution (OOD) images with a generative adversarial network (GAN). Our training dataset was comprised of 3,234 liver-containing computed tomography (CT) scans from 456 patients. Our OOD test data consisted of CT images of the brain, head and neck, lung, cervix, and abnormal livers. A StyleGAN2-ADA architecture was employed to model the training distribution. Images were reconstructed using backpropagation. Reconstructions were evaluated using the Wasserstein distance, mean squared error, and the structural similarity index measure. OOD detection was evaluated with the area under the receiver operating characteristic curve (AUROC). Our paradigm distinguished between liver and non-liver CT with greater than 90% AUROC. It was also completely unable to reconstruct liver artifacts, such as needles and ascites.
* Extended abstract published in the "Medical Imaging Meets NeurIPS" workshop at NeurIPS 2022. Original abstract can be found at http://www.cse.cuhk.edu.hk/~qdou/public/medneurips2022/125.pdf
Evaluating the Performance of StyleGAN2-ADA on Medical Images
Oct 07, 2022Abstract:Although generative adversarial networks (GANs) have shown promise in medical imaging, they have four main limitations that impeded their utility: computational cost, data requirements, reliable evaluation measures, and training complexity. Our work investigates each of these obstacles in a novel application of StyleGAN2-ADA to high-resolution medical imaging datasets. Our dataset is comprised of liver-containing axial slices from non-contrast and contrast-enhanced computed tomography (CT) scans. Additionally, we utilized four public datasets composed of various imaging modalities. We trained a StyleGAN2 network with transfer learning (from the Flickr-Faces-HQ dataset) and data augmentation (horizontal flipping and adaptive discriminator augmentation). The network's generative quality was measured quantitatively with the Fr\'echet Inception Distance (FID) and qualitatively with a visual Turing test given to seven radiologists and radiation oncologists. The StyleGAN2-ADA network achieved a FID of 5.22 ($\pm$ 0.17) on our liver CT dataset. It also set new record FIDs of 10.78, 3.52, 21.17, and 5.39 on the publicly available SLIVER07, ChestX-ray14, ACDC, and Medical Segmentation Decathlon (brain tumors) datasets. In the visual Turing test, the clinicians rated generated images as real 42% of the time, approaching random guessing. Our computational ablation study revealed that transfer learning and data augmentation stabilize training and improve the perceptual quality of the generated images. We observed the FID to be consistent with human perceptual evaluation of medical images. Finally, our work found that StyleGAN2-ADA consistently produces high-quality results without hyperparameter searches or retraining.
* This preprint has not undergone post-submission improvements or corrections. The Version of Record of this contribution is published in LNCS, volume 13570, and is available online at https://doi.org/10.1007/978-3-031-16980-9_14
Integrative Imaging Informatics for Cancer Research: Workflow Automation for Neuro-oncology (I3CR-WANO)
Oct 06, 2022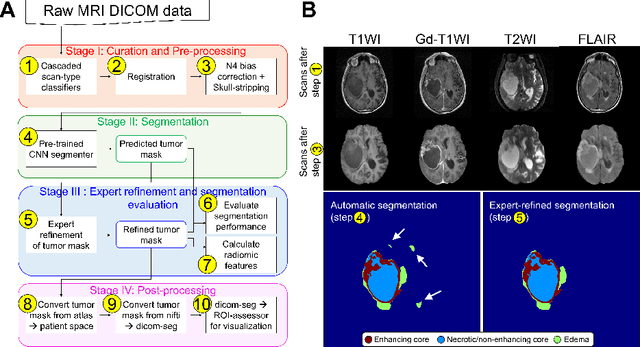
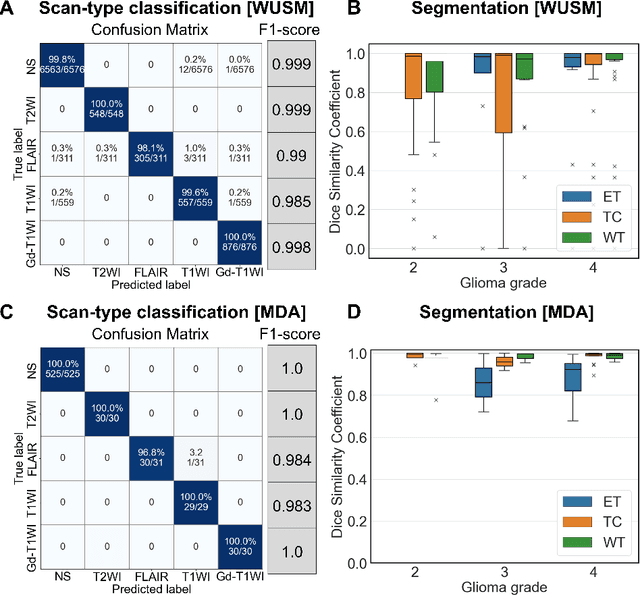
Abstract:Efforts to utilize growing volumes of clinical imaging data to generate tumor evaluations continue to require significant manual data wrangling owing to the data heterogeneity. Here, we propose an artificial intelligence-based solution for the aggregation and processing of multisequence neuro-oncology MRI data to extract quantitative tumor measurements. Our end-to-end framework i) classifies MRI sequences using an ensemble classifier, ii) preprocesses the data in a reproducible manner, iii) delineates tumor tissue subtypes using convolutional neural networks, and iv) extracts diverse radiomic features. Moreover, it is robust to missing sequences and adopts an expert-in-the-loop approach, where the segmentation results may be manually refined by radiologists. Following the implementation of the framework in Docker containers, it was applied to two retrospective glioma datasets collected from the Washington University School of Medicine (WUSM; n = 384) and the M.D. Anderson Cancer Center (MDA; n = 30) comprising preoperative MRI scans from patients with pathologically confirmed gliomas. The scan-type classifier yielded an accuracy of over 99%, correctly identifying sequences from 380/384 and 30/30 sessions from the WUSM and MDA datasets, respectively. Segmentation performance was quantified using the Dice Similarity Coefficient between the predicted and expert-refined tumor masks. Mean Dice scores were 0.882 ($\pm$0.244) and 0.977 ($\pm$0.04) for whole tumor segmentation for WUSM and MDA, respectively. This streamlined framework automatically curated, processed, and segmented raw MRI data of patients with varying grades of gliomas, enabling the curation of large-scale neuro-oncology datasets and demonstrating a high potential for integration as an assistive tool in clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge