Michael B. Gotway
Lamps: Learning Anatomy from Multiple Perspectives via Self-supervision in Chest Radiographs
Jan 02, 2026Abstract:Foundation models have been successful in natural language processing and computer vision because they are capable of capturing the underlying structures (foundation) of natural languages. However, in medical imaging, the key foundation lies in human anatomy, as these images directly represent the internal structures of the body, reflecting the consistency, coherence, and hierarchy of human anatomy. Yet, existing self-supervised learning (SSL) methods often overlook these perspectives, limiting their ability to effectively learn anatomical features. To overcome the limitation, we built Lamps (learning anatomy from multiple perspectives via self-supervision) pre-trained on large-scale chest radiographs by harmoniously utilizing the consistency, coherence, and hierarchy of human anatomy as the supervision signal. Extensive experiments across 10 datasets evaluated through fine-tuning and emergent property analysis demonstrate Lamps' superior robustness, transferability, and clinical potential when compared to 10 baseline models. By learning from multiple perspectives, Lamps presents a unique opportunity for foundation models to develop meaningful, robust representations that are aligned with the structure of human anatomy.
Learning Anatomy from Multiple Perspectives via Self-supervision in Chest Radiographs
Dec 28, 2025Abstract:Foundation models have been successful in natural language processing and computer vision because they are capable of capturing the underlying structures (foundation) of natural languages. However, in medical imaging, the key foundation lies in human anatomy, as these images directly represent the internal structures of the body, reflecting the consistency, coherence, and hierarchy of human anatomy. Yet, existing self-supervised learning (SSL) methods often overlook these perspectives, limiting their ability to effectively learn anatomical features. To overcome the limitation, we built Lamps (learning anatomy from multiple perspectives via self-supervision) pre-trained on large-scale chest radiographs by harmoniously utilizing the consistency, coherence, and hierarchy of human anatomy as the supervision signal. Extensive experiments across 10 datasets evaluated through fine-tuning and emergent property analysis demonstrate Lamps' superior robustness, transferability, and clinical potential when compared to 10 baseline models. By learning from multiple perspectives, Lamps presents a unique opportunity for foundation models to develop meaningful, robust representations that are aligned with the structure of human anatomy.
Representing Part-Whole Hierarchies in Foundation Models by Learning Localizability, Composability, and Decomposability from Anatomy via Self-Supervision
Apr 24, 2024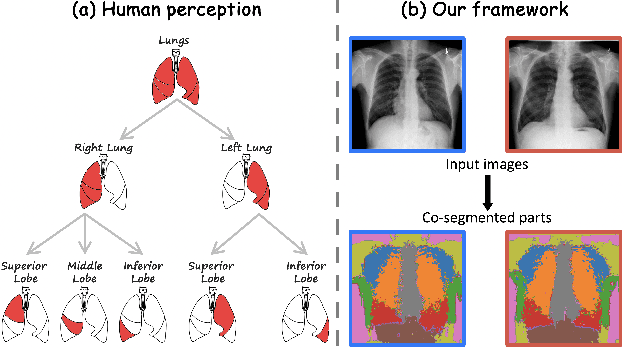
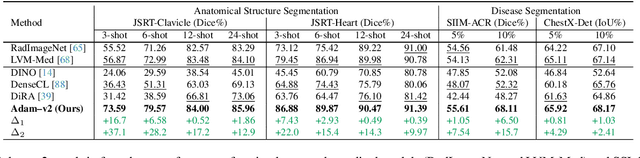
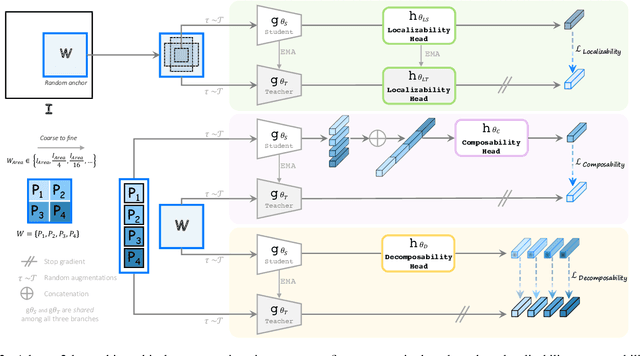
Abstract:Humans effortlessly interpret images by parsing them into part-whole hierarchies; deep learning excels in learning multi-level feature spaces, but they often lack explicit coding of part-whole relations, a prominent property of medical imaging. To overcome this limitation, we introduce Adam-v2, a new self-supervised learning framework extending Adam [79] by explicitly incorporating part-whole hierarchies into its learning objectives through three key branches: (1) Localizability, acquiring discriminative representations to distinguish different anatomical patterns; (2) Composability, learning each anatomical structure in a parts-to-whole manner; and (3) Decomposability, comprehending each anatomical structure in a whole-to-parts manner. Experimental results across 10 tasks, compared to 11 baselines in zero-shot, few-shot transfer, and full fine-tuning settings, showcase Adam-v2's superior performance over large-scale medical models and existing SSL methods across diverse downstream tasks. The higher generality and robustness of Adam-v2's representations originate from its explicit construction of hierarchies for distinct anatomical structures from unlabeled medical images. Adam-v2 preserves a semantic balance of anatomical diversity and harmony in its embedding, yielding representations that are both generic and semantically meaningful, yet overlooked in existing SSL methods. All code and pretrained models are available at https://github.com/JLiangLab/Eden.
Foundation Ark: Accruing and Reusing Knowledge for Superior and Robust Performance
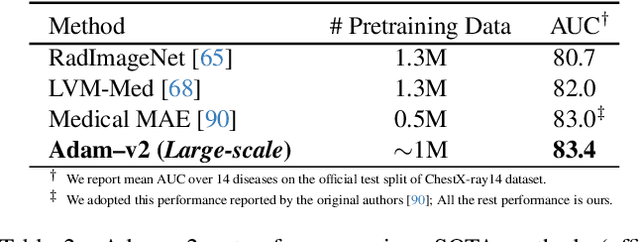
Oct 14, 2023Abstract:Deep learning nowadays offers expert-level and sometimes even super-expert-level performance, but achieving such performance demands massive annotated data for training (e.g., Google's proprietary CXR Foundation Model (CXR-FM) was trained on 821,544 labeled and mostly private chest X-rays (CXRs)). Numerous datasets are publicly available in medical imaging but individually small and heterogeneous in expert labels. We envision a powerful and robust foundation model that can be trained by aggregating numerous small public datasets. To realize this vision, we have developed Ark, a framework that accrues and reuses knowledge from heterogeneous expert annotations in various datasets. As a proof of concept, we have trained two Ark models on 335,484 and 704,363 CXRs, respectively, by merging several datasets including ChestX-ray14, CheXpert, MIMIC-II, and VinDr-CXR, evaluated them on a wide range of imaging tasks covering both classification and segmentation via fine-tuning, linear-probing, and gender-bias analysis, and demonstrated our Ark's superior and robust performance over the SOTA fully/self-supervised baselines and Google's proprietary CXR-FM. This enhanced performance is attributed to our simple yet powerful observation that aggregating numerous public datasets diversifies patient populations and accrues knowledge from diverse experts, yielding unprecedented performance yet saving annotation cost. With all codes and pretrained models released at GitHub.com/JLiangLab/Ark, we hope that Ark exerts an important impact on open science, as accruing and reusing knowledge from expert annotations in public datasets can potentially surpass the performance of proprietary models trained on unusually large data, inspiring many more researchers worldwide to share codes and datasets to build open foundation models, accelerate open science, and democratize deep learning for medical imaging.
Towards Foundation Models Learned from Anatomy in Medical Imaging via Self-Supervision
Sep 27, 2023Abstract:Human anatomy is the foundation of medical imaging and boasts one striking characteristic: its hierarchy in nature, exhibiting two intrinsic properties: (1) locality: each anatomical structure is morphologically distinct from the others; and (2) compositionality: each anatomical structure is an integrated part of a larger whole. We envision a foundation model for medical imaging that is consciously and purposefully developed upon this foundation to gain the capability of "understanding" human anatomy and to possess the fundamental properties of medical imaging. As our first step in realizing this vision towards foundation models in medical imaging, we devise a novel self-supervised learning (SSL) strategy that exploits the hierarchical nature of human anatomy. Our extensive experiments demonstrate that the SSL pretrained model, derived from our training strategy, not only outperforms state-of-the-art (SOTA) fully/self-supervised baselines but also enhances annotation efficiency, offering potential few-shot segmentation capabilities with performance improvements ranging from 9% to 30% for segmentation tasks compared to SSL baselines. This performance is attributed to the significance of anatomy comprehension via our learning strategy, which encapsulates the intrinsic attributes of anatomical structures-locality and compositionality-within the embedding space, yet overlooked in existing SSL methods. All code and pretrained models are available at https://github.com/JLiangLab/Eden.
DiRA: Discriminative, Restorative, and Adversarial Learning for Self-supervised Medical Image Analysis
Apr 21, 2022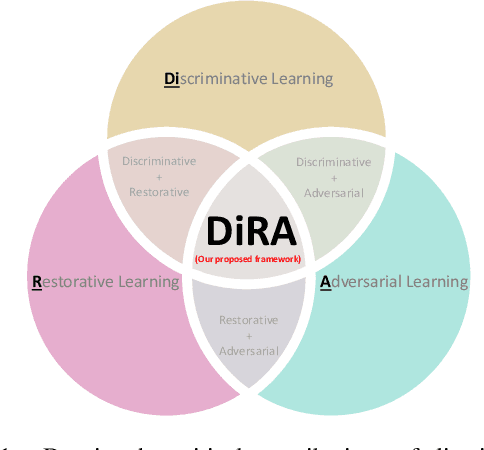
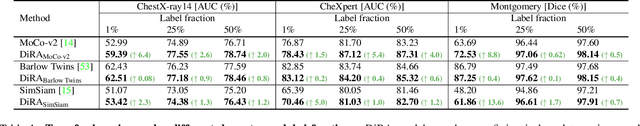
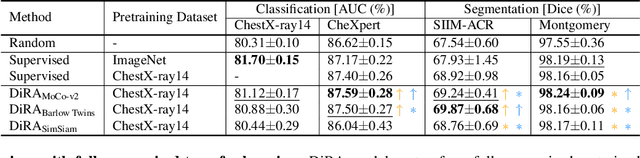
Abstract:Discriminative learning, restorative learning, and adversarial learning have proven beneficial for self-supervised learning schemes in computer vision and medical imaging. Existing efforts, however, omit their synergistic effects on each other in a ternary setup, which, we envision, can significantly benefit deep semantic representation learning. To realize this vision, we have developed DiRA, the first framework that unites discriminative, restorative, and adversarial learning in a unified manner to collaboratively glean complementary visual information from unlabeled medical images for fine-grained semantic representation learning. Our extensive experiments demonstrate that DiRA (1) encourages collaborative learning among three learning ingredients, resulting in more generalizable representation across organs, diseases, and modalities; (2) outperforms fully supervised ImageNet models and increases robustness in small data regimes, reducing annotation cost across multiple medical imaging applications; (3) learns fine-grained semantic representation, facilitating accurate lesion localization with only image-level annotation; and (4) enhances state-of-the-art restorative approaches, revealing that DiRA is a general mechanism for united representation learning. All code and pre-trained models are available at https: //github.com/JLiangLab/DiRA.
CAiD: Context-Aware Instance Discrimination for Self-supervised Learning in Medical Imaging
Apr 15, 2022
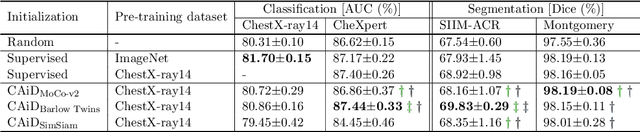
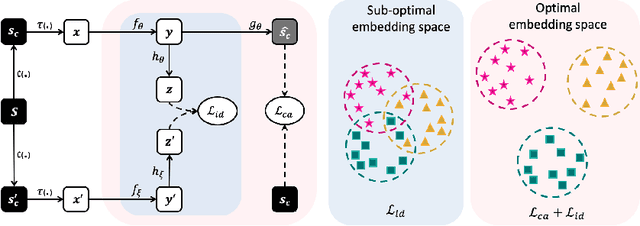
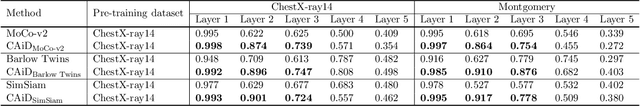
Abstract:Recently, self-supervised instance discrimination methods have achieved significant success in learning visual representations from unlabeled photographic images. However, given the marked differences between photographic and medical images, the efficacy of instance-based objectives, focusing on learning the most discriminative global features in the image (i.e., wheels in bicycle), remains unknown in medical imaging. Our preliminary analysis showed that high global similarity of medical images in terms of anatomy hampers instance discrimination methods for capturing a set of distinct features, negatively impacting their performance on medical downstream tasks. To alleviate this limitation, we have developed a simple yet effective self-supervised framework, called Context-Aware instance Discrimination (CAiD). CAiD aims to improve instance discrimination learning by providing finer and more discriminative information encoded from a diverse local context of unlabeled medical images. We conduct a systematic analysis to investigate the utility of the learned features from a three-pronged perspective: (i) generalizability and transferability, (ii) separability in the embedding space, and (iii) reusability. Our extensive experiments demonstrate that CAiD (1) enriches representations learned from existing instance discrimination methods; (2) delivers more discriminative features by adequately capturing finer contextual information from individual medial images; and (3) improves reusability of low/mid-level features compared to standard instance discriminative methods. As open science, all codes and pre-trained models are available on our GitHub page: https://github.com/JLiangLab/CAiD.
A Systematic Benchmarking Analysis of Transfer Learning for Medical Image Analysis
Aug 12, 2021
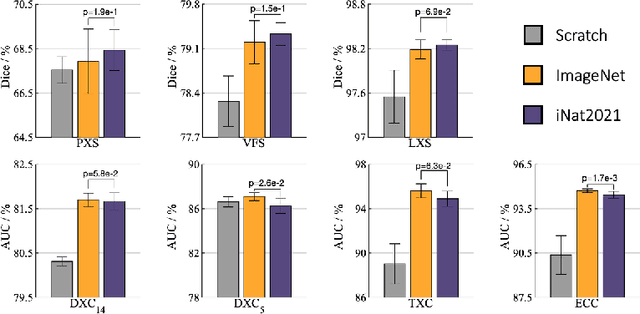
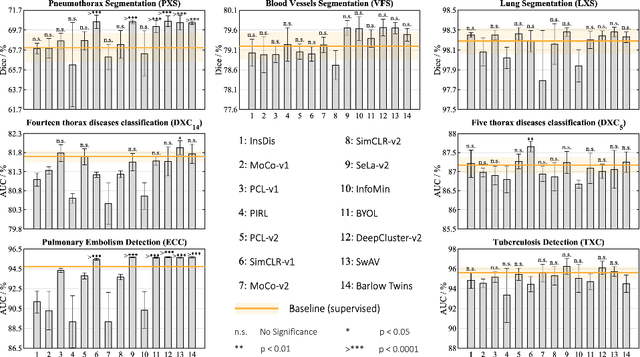
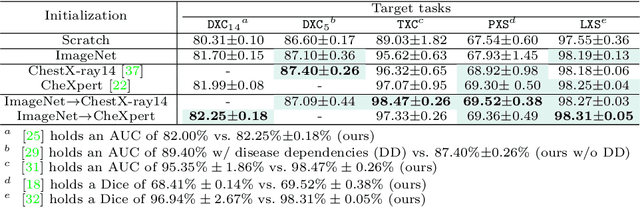
Abstract:Transfer learning from supervised ImageNet models has been frequently used in medical image analysis. Yet, no large-scale evaluation has been conducted to benchmark the efficacy of newly-developed pre-training techniques for medical image analysis, leaving several important questions unanswered. As the first step in this direction, we conduct a systematic study on the transferability of models pre-trained on iNat2021, the most recent large-scale fine-grained dataset, and 14 top self-supervised ImageNet models on 7 diverse medical tasks in comparison with the supervised ImageNet model. Furthermore, we present a practical approach to bridge the domain gap between natural and medical images by continually (pre-)training supervised ImageNet models on medical images. Our comprehensive evaluation yields new insights: (1) pre-trained models on fine-grained data yield distinctive local representations that are more suitable for medical segmentation tasks, (2) self-supervised ImageNet models learn holistic features more effectively than supervised ImageNet models, and (3) continual pre-training can bridge the domain gap between natural and medical images. We hope that this large-scale open evaluation of transfer learning can direct the future research of deep learning for medical imaging. As open science, all codes and pre-trained models are available on our GitHub page https://github.com/JLiangLab/BenchmarkTransferLearning.
Transferable Visual Words: Exploiting the Semantics of Anatomical Patterns for Self-supervised Learning
Feb 21, 2021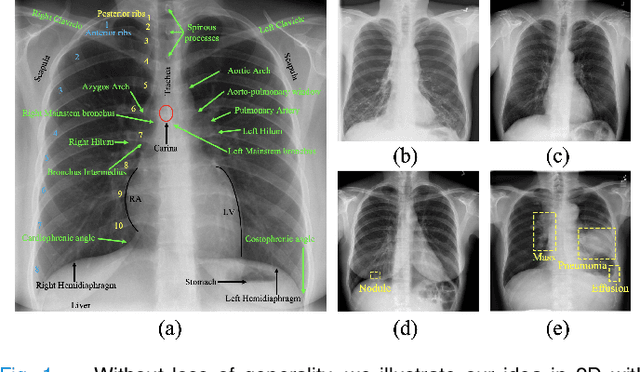
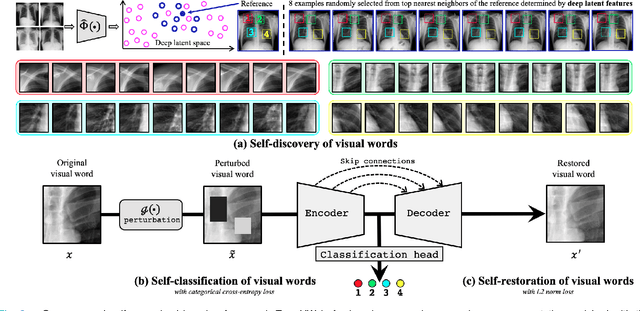
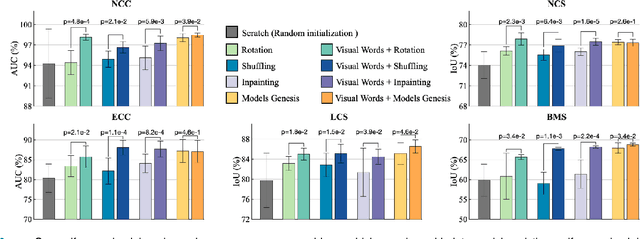
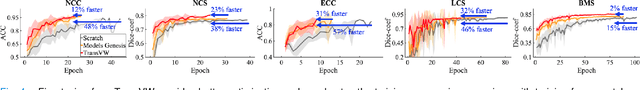
Abstract:This paper introduces a new concept called "transferable visual words" (TransVW), aiming to achieve annotation efficiency for deep learning in medical image analysis. Medical imaging--focusing on particular parts of the body for defined clinical purposes--generates images of great similarity in anatomy across patients and yields sophisticated anatomical patterns across images, which are associated with rich semantics about human anatomy and which are natural visual words. We show that these visual words can be automatically harvested according to anatomical consistency via self-discovery, and that the self-discovered visual words can serve as strong yet free supervision signals for deep models to learn semantics-enriched generic image representation via self-supervision (self-classification and self-restoration). Our extensive experiments demonstrate the annotation efficiency of TransVW by offering higher performance and faster convergence with reduced annotation cost in several applications. Our TransVW has several important advantages, including (1) TransVW is a fully autodidactic scheme, which exploits the semantics of visual words for self-supervised learning, requiring no expert annotation; (2) visual word learning is an add-on strategy, which complements existing self-supervised methods, boosting their performance; and (3) the learned image representation is semantics-enriched models, which have proven to be more robust and generalizable, saving annotation efforts for a variety of applications through transfer learning. Our code, pre-trained models, and curated visual words are available at https://github.com/JLiangLab/TransVW.
Learning Semantics-enriched Representation via Self-discovery, Self-classification, and Self-restoration
Jul 14, 2020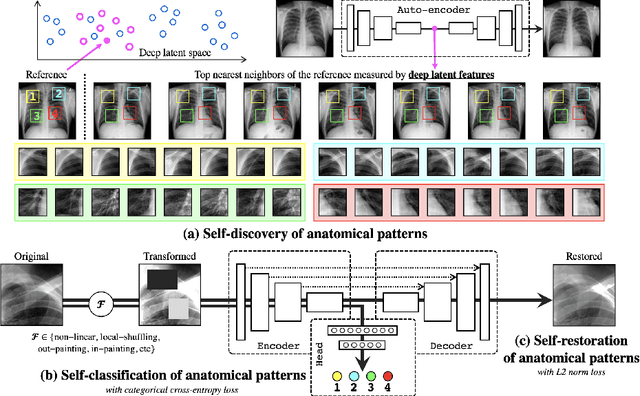
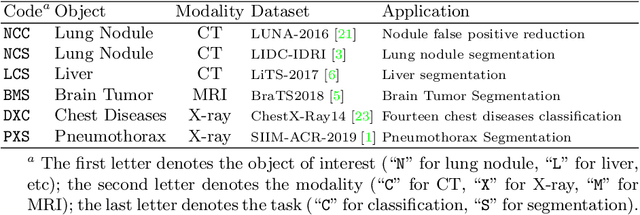
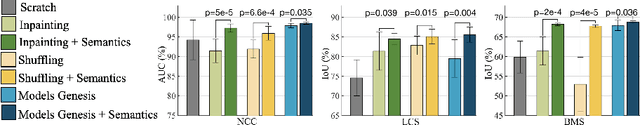
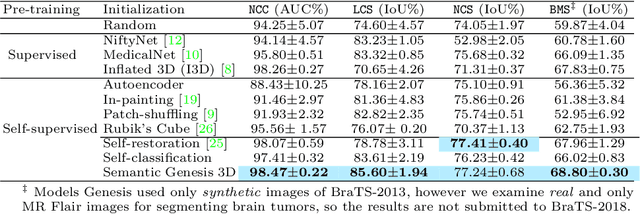
Abstract:Medical images are naturally associated with rich semantics about the human anatomy, reflected in an abundance of recurring anatomical patterns, offering unique potential to foster deep semantic representation learning and yield semantically more powerful models for different medical applications. But how exactly such strong yet free semantics embedded in medical images can be harnessed for self-supervised learning remains largely unexplored. To this end, we train deep models to learn semantically enriched visual representation by self-discovery, self-classification, and self-restoration of the anatomy underneath medical images, resulting in a semantics-enriched, general-purpose, pre-trained 3D model, named Semantic Genesis. We examine our Semantic Genesis with all the publicly-available pre-trained models, by either self-supervision or fully supervision, on the six distinct target tasks, covering both classification and segmentation in various medical modalities (i.e.,CT, MRI, and X-ray). Our extensive experiments demonstrate that Semantic Genesis significantly exceeds all of its 3D counterparts as well as the de facto ImageNet-based transfer learning in 2D. This performance is attributed to our novel self-supervised learning framework, encouraging deep models to learn compelling semantic representation from abundant anatomical patterns resulting from consistent anatomies embedded in medical images. Code and pre-trained Semantic Genesis are available at https://github.com/JLiangLab/SemanticGenesis .
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge