Marius Distler
Mission Balance: Generating Under-represented Class Samples using Video Diffusion Models
May 14, 2025Abstract:Computer-assisted interventions can improve intra-operative guidance, particularly through deep learning methods that harness the spatiotemporal information in surgical videos. However, the severe data imbalance often found in surgical video datasets hinders the development of high-performing models. In this work, we aim to overcome the data imbalance by synthesizing surgical videos. We propose a unique two-stage, text-conditioned diffusion-based method to generate high-fidelity surgical videos for under-represented classes. Our approach conditions the generation process on text prompts and decouples spatial and temporal modeling by utilizing a 2D latent diffusion model to capture spatial content and then integrating temporal attention layers to ensure temporal consistency. Furthermore, we introduce a rejection sampling strategy to select the most suitable synthetic samples, effectively augmenting existing datasets to address class imbalance. We evaluate our method on two downstream tasks-surgical action recognition and intra-operative event prediction-demonstrating that incorporating synthetic videos from our approach substantially enhances model performance. We open-source our implementation at https://gitlab.com/nct_tso_public/surgvgen.
One model to use them all: Training a segmentation model with complementary datasets
Feb 29, 2024Abstract:Understanding a surgical scene is crucial for computer-assisted surgery systems to provide any intelligent assistance functionality. One way of achieving this scene understanding is via scene segmentation, where every pixel of a frame is classified and therefore identifies the visible structures and tissues. Progress on fully segmenting surgical scenes has been made using machine learning. However, such models require large amounts of annotated training data, containing examples of all relevant object classes. Such fully annotated datasets are hard to create, as every pixel in a frame needs to be annotated by medical experts and, therefore, are rarely available. In this work, we propose a method to combine multiple partially annotated datasets, which provide complementary annotations, into one model, enabling better scene segmentation and the use of multiple readily available datasets. Our method aims to combine available data with complementary labels by leveraging mutual exclusive properties to maximize information. Specifically, we propose to use positive annotations of other classes as negative samples and to exclude background pixels of binary annotations, as we cannot tell if they contain a class not annotated but predicted by the model. We evaluate our method by training a DeepLabV3 on the publicly available Dresden Surgical Anatomy Dataset, which provides multiple subsets of binary segmented anatomical structures. Our approach successfully combines 6 classes into one model, increasing the overall Dice Score by 4.4% compared to an ensemble of models trained on the classes individually. By including information on multiple classes, we were able to reduce confusion between stomach and colon by 24%. Our results demonstrate the feasibility of training a model on multiple datasets. This paves the way for future work further alleviating the need for one large, fully segmented datasets.
Exploring Semantic Consistency in Unpaired Image Translation to Generate Data for Surgical Applications
Sep 11, 2023Abstract:In surgical computer vision applications, obtaining labeled training data is challenging due to data-privacy concerns and the need for expert annotation. Unpaired image-to-image translation techniques have been explored to automatically generate large annotated datasets by translating synthetic images to the realistic domain. However, preserving the structure and semantic consistency between the input and translated images presents significant challenges, mainly when there is a distributional mismatch in the semantic characteristics of the domains. This study empirically investigates unpaired image translation methods for generating suitable data in surgical applications, explicitly focusing on semantic consistency. We extensively evaluate various state-of-the-art image translation models on two challenging surgical datasets and downstream semantic segmentation tasks. We find that a simple combination of structural-similarity loss and contrastive learning yields the most promising results. Quantitatively, we show that the data generated with this approach yields higher semantic consistency and can be used more effectively as training data.
Rethinking Anticipation Tasks: Uncertainty-aware Anticipation of Sparse Surgical Instrument Usage for Context-aware Assistance
Jul 16, 2020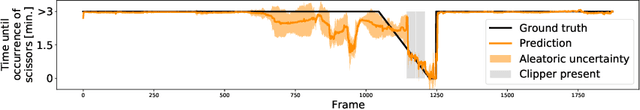
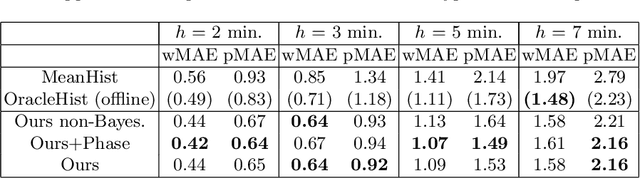
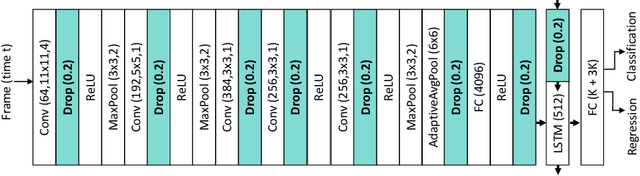
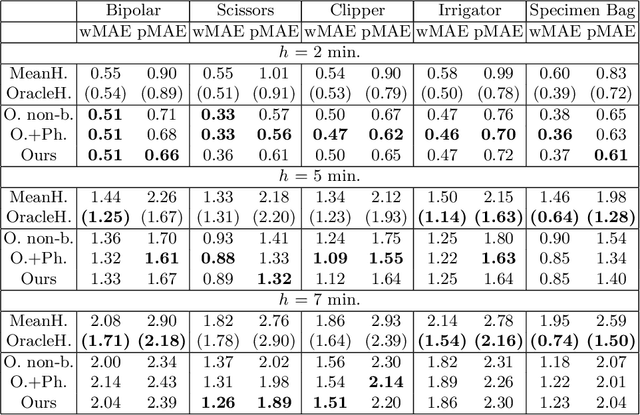
Abstract:Intra-operative anticipation of instrument usage is a necessary component for context-aware assistance in surgery, e.g. for instrument preparation or semi-automation of robotic tasks. However, the sparsity of instrument occurrences in long videos poses a challenge. Current approaches are limited as they assume knowledge on the timing of future actions or require dense temporal segmentations during training and inference. We propose a novel learning task for anticipation of instrument usage in laparoscopic videos that overcomes these limitations. During training, only sparse instrument annotations are required and inference is done solely on image data. We train a probabilistic model to address the uncertainty associated with future events. Our approach outperforms several baselines and is competitive to a variant using richer annotations. We demonstrate the model's ability to quantify task-relevant uncertainties. To the best of our knowledge, we are the first to propose a method for anticipating instruments in surgery.
Unsupervised Temporal Video Segmentation as an Auxiliary Task for Predicting the Remaining Surgery Duration
Feb 26, 2020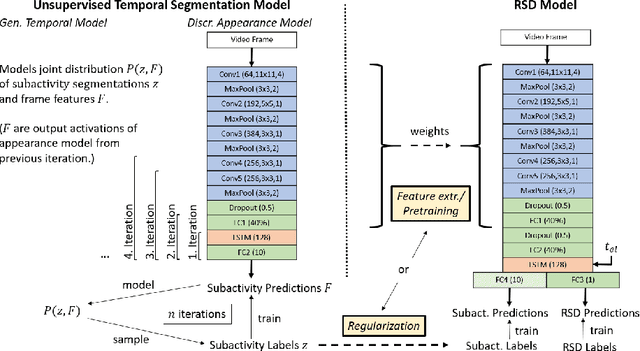



Abstract:Estimating the remaining surgery duration (RSD) during surgical procedures can be useful for OR planning and anesthesia dose estimation. With the recent success of deep learning-based methods in computer vision, several neural network approaches have been proposed for fully automatic RSD prediction based solely on visual data from the endoscopic camera. We investigate whether RSD prediction can be improved using unsupervised temporal video segmentation as an auxiliary learning task. As opposed to previous work, which presented supervised surgical phase recognition as auxiliary task, we avoid the need for manual annotations by proposing a similar but unsupervised learning objective which clusters video sequences into temporally coherent segments. In multiple experimental setups, results obtained by learning the auxiliary task are incorporated into a deep RSD model through feature extraction, pretraining or regularization. Further, we propose a novel loss function for RSD training which attempts to counteract unfavorable characteristics of the RSD ground truth. Using our unsupervised method as an auxiliary task for RSD training, we outperform other self-supervised methods and are comparable to the supervised state-of-the-art. Combined with the novel RSD loss, we slightly outperform the supervised approach.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge