Maria del C. Valdés Hernández
Pre-processing and quality control of large clinical CT head datasets for intracranial arterial calcification segmentation
Aug 02, 2024Abstract:As a potential non-invasive biomarker for ischaemic stroke, intracranial arterial calcification (IAC) could be used for stroke risk assessment on CT head scans routinely acquired for other reasons (e.g. trauma, confusion). Artificial intelligence methods can support IAC scoring, but they have not yet been developed for clinical imaging. Large heterogeneous clinical CT datasets are necessary for the training of such methods, but they exhibit expected and unexpected data anomalies. Using CTs from a large clinical trial, the third International Stroke Trial (IST-3), we propose a pipeline that uses as input non-enhanced CT scans to output regions of interest capturing selected large intracranial arteries for IAC scoring. Our method uses co-registration with templates. We focus on quality control, using information presence along the z-axis of the imaging to group and apply similarity measures (structural similarity index measure) to triage assessment of individual image series. Additionally, we propose superimposing thresholded binary masks of the series to inspect large quantities of data in parallel. We identify and exclude unrecoverable samples and registration failures. In total, our pipeline processes 10,659 CT series, rejecting 4,322 (41%) in the entire process, 1,450 (14% of the total) during quality control, and outputting 6,337 series. Our pipeline enables effective and efficient region of interest localisation for targeted IAC segmentation.
Quality control for more reliable integration of deep learning-based image segmentation into medical workflows
Dec 06, 2021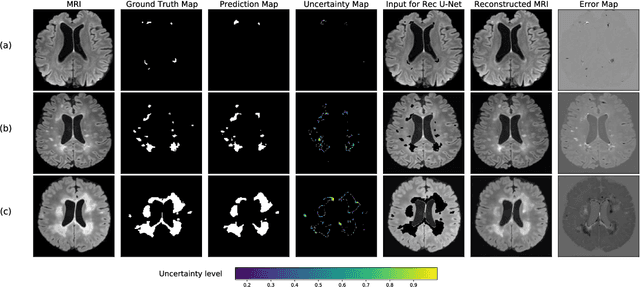
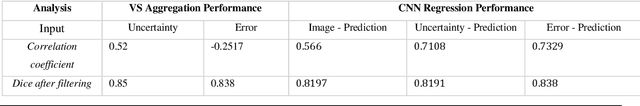
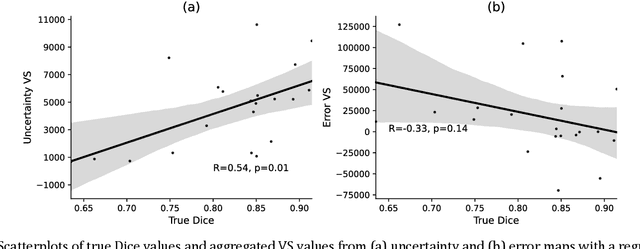
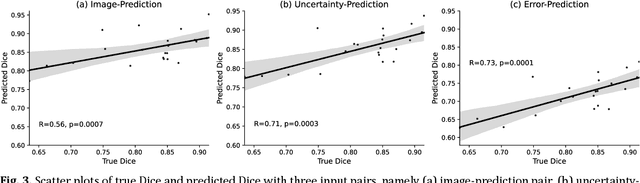
Abstract:Machine learning algorithms underpin modern diagnostic-aiding software, which has proved valuable in clinical practice, particularly in radiology. However, inaccuracies, mainly due to the limited availability of clinical samples for training these algorithms, hamper their wider applicability, acceptance, and recognition amongst clinicians. We present an analysis of state-of-the-art automatic quality control (QC) approaches that can be implemented within these algorithms to estimate the certainty of their outputs. We validated the most promising approaches on a brain image segmentation task identifying white matter hyperintensities (WMH) in magnetic resonance imaging data. WMH are a correlate of small vessel disease common in mid-to-late adulthood and are particularly challenging to segment due to their varied size, and distributional patterns. Our results show that the aggregation of uncertainty and Dice prediction were most effective in failure detection for this task. Both methods independently improved mean Dice from 0.82 to 0.84. Our work reveals how QC methods can help to detect failed segmentation cases and therefore make automatic segmentation more reliable and suitable for clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge