Paul Armitage
Pre-processing and quality control of large clinical CT head datasets for intracranial arterial calcification segmentation
Aug 02, 2024Abstract:As a potential non-invasive biomarker for ischaemic stroke, intracranial arterial calcification (IAC) could be used for stroke risk assessment on CT head scans routinely acquired for other reasons (e.g. trauma, confusion). Artificial intelligence methods can support IAC scoring, but they have not yet been developed for clinical imaging. Large heterogeneous clinical CT datasets are necessary for the training of such methods, but they exhibit expected and unexpected data anomalies. Using CTs from a large clinical trial, the third International Stroke Trial (IST-3), we propose a pipeline that uses as input non-enhanced CT scans to output regions of interest capturing selected large intracranial arteries for IAC scoring. Our method uses co-registration with templates. We focus on quality control, using information presence along the z-axis of the imaging to group and apply similarity measures (structural similarity index measure) to triage assessment of individual image series. Additionally, we propose superimposing thresholded binary masks of the series to inspect large quantities of data in parallel. We identify and exclude unrecoverable samples and registration failures. In total, our pipeline processes 10,659 CT series, rejecting 4,322 (41%) in the entire process, 1,450 (14% of the total) during quality control, and outputting 6,337 series. Our pipeline enables effective and efficient region of interest localisation for targeted IAC segmentation.
Development of a Deep Learning Method to Identify Acute Ischemic Stroke Lesions on Brain CT
Sep 29, 2023



Abstract:Computed Tomography (CT) is commonly used to image acute ischemic stroke (AIS) patients, but its interpretation by radiologists is time-consuming and subject to inter-observer variability. Deep learning (DL) techniques can provide automated CT brain scan assessment, but usually require annotated images. Aiming to develop a DL method for AIS using labelled but not annotated CT brain scans from patients with AIS, we designed a convolutional neural network-based DL algorithm using routinely-collected CT brain scans from the Third International Stroke Trial (IST-3), which were not acquired using strict research protocols. The DL model aimed to detect AIS lesions and classify the side of the brain affected. We explored the impact of AIS lesion features, background brain appearances, and timing on DL performance. From 5772 unique CT scans of 2347 AIS patients (median age 82), 54% had visible AIS lesions according to expert labelling. Our best-performing DL method achieved 72% accuracy for lesion presence and side. Lesions that were larger (80% accuracy) or multiple (87% accuracy for two lesions, 100% for three or more), were better detected. Follow-up scans had 76% accuracy, while baseline scans 67% accuracy. Chronic brain conditions reduced accuracy, particularly non-stroke lesions and old stroke lesions (32% and 31% error rates respectively). DL methods can be designed for AIS lesion detection on CT using the vast quantities of routinely-collected CT brain scan data. Ultimately, this should lead to more robust and widely-applicable methods.
Challenges of building medical image datasets for development of deep learning software in stroke
Sep 26, 2023Abstract:Despite the large amount of brain CT data generated in clinical practice, the availability of CT datasets for deep learning (DL) research is currently limited. Furthermore, the data can be insufficiently or improperly prepared for machine learning and thus lead to spurious and irreproducible analyses. This lack of access to comprehensive and diverse datasets poses a significant challenge for the development of DL algorithms. In this work, we propose a complete semi-automatic pipeline to address the challenges of preparing a clinical brain CT dataset for DL analysis and describe the process of standardising this heterogeneous dataset. Challenges include handling image sets with different orientations (axial, sagittal, coronal), different image types (to view soft tissues or bones) and dimensions, and removing redundant background. The final pipeline was able to process 5,868/10,659 (45%) CT image datasets. Reasons for rejection include non-axial data (n=1,920), bone reformats (n=687), separated skull base/vault images (n=1,226), and registration failures (n=465). Further format adjustments, including image cropping, resizing and scaling are also needed for DL processing. Of the axial scans that were not localisers, bone reformats or split brains, 5,868/6,333 (93%) were accepted, while the remaining 465 failed the registration process. Appropriate preparation of medical imaging datasets for DL is a costly and time-intensive process.
Spatio-Angular Convolutions for Super-resolution in Diffusion MRI
Jun 01, 2023



Abstract:Diffusion MRI (dMRI) is a widely used imaging modality, but requires long scanning times to acquire high resolution datasets. By leveraging the unique geometry present within this domain, we present a novel approach to dMRI angular super-resolution that extends upon the parametric continuous convolution (PCConv) framework. We introduce several additions to the operation including a Fourier feature mapping, global coordinates, and domain specific context. Using this framework, we build a fully parametric continuous convolution network (PCCNN) and compare against existing models. We demonstrate the PCCNN performs competitively while using significantly less parameters. Moreover, we show that this formulation generalises well to clinically relevant downstream analyses such as fixel-based analysis, and neurite orientation dispersion and density imaging.
Angular Super-Resolution in Diffusion MRI with a 3D Recurrent Convolutional Autoencoder
Mar 29, 2022

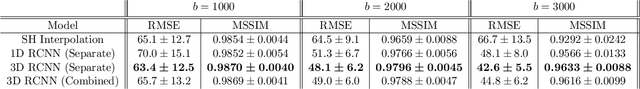
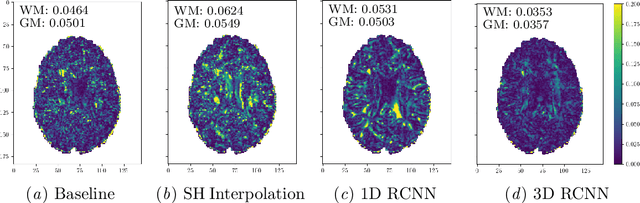
Abstract:High resolution diffusion MRI (dMRI) data is often constrained by limited scanning time in clinical settings, thus restricting the use of downstream analysis techniques that would otherwise be available. In this work we develop a 3D recurrent convolutional neural network (RCNN) capable of super-resolving dMRI volumes in the angular (q-space) domain. Our approach formulates the task of angular super-resolution as a patch-wise regression using a 3D autoencoder conditioned on target b-vectors. Within the network we use a convolutional long short term memory (ConvLSTM) cell to model the relationship between q-space samples. We compare model performance against a baseline spherical harmonic interpolation and a 1D variant of the model architecture. We show that the 3D model has the lowest error rates across different subsampling schemes and b-values. The relative performance of the 3D RCNN is greatest in the very low angular resolution domain. Code for this project is available at https://github.com/m-lyon/dMRI-RCNN.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge