Manuel Schultheiss
1-3
Towards AI Lesion Tracking in PET/CT Imaging: A Siamese-based CNN Pipeline applied on PSMA PET/CT Scans
Jun 13, 2024



Abstract:Assessing tumor response to systemic therapies is one of the main applications of PET/CT. Routinely, only a small subset of index lesions out of multiple lesions is analyzed. However, this operator dependent selection may bias the results due to possible significant inter-metastatic heterogeneity of response to therapy. Automated, AI based approaches for lesion tracking hold promise in enabling the analysis of many more lesions and thus providing a better assessment of tumor response. This work introduces a Siamese CNN approach for lesion tracking between PET/CT scans. Our approach is applied on the laborious task of tracking a high number of bone lesions in full-body baseline and follow-up [68Ga]Ga- or [18F]F-PSMA PET/CT scans after two cycles of [177Lu]Lu-PSMA therapy of metastatic castration resistant prostate cancer patients. Data preparation includes lesion segmentation and affine registration. Our algorithm extracts suitable lesion patches and forwards them into a Siamese CNN trained to classify the lesion patch pairs as corresponding or non-corresponding lesions. Experiments have been performed with different input patch types and a Siamese network in 2D and 3D. The CNN model successfully learned to classify lesion assignments, reaching a lesion tracking accuracy of 83 % in its best configuration with an AUC = 0.91. For remaining lesions the pipeline accomplished a re-identification rate of 89 %. We proved that a CNN may facilitate the tracking of multiple lesions in PSMA PET/CT scans. Future clinical studies are necessary if this improves the prediction of the outcome of therapies.
Improving Automated Hemorrhage Detection in Sparse-view Computed Tomography via Deep Convolutional Neural Network based Artifact Reduction
Mar 16, 2023Abstract:Intracranial hemorrhage poses a serious health problem requiring rapid and often intensive medical treatment. For diagnosis, a Cranial Computed Tomography (CCT) scan is usually performed. However, the increased health risk caused by radiation is a concern. The most important strategy to reduce this potential risk is to keep the radiation dose as low as possible and consistent with the diagnostic task. Sparse-view CT can be an effective strategy to reduce dose by reducing the total number of views acquired, albeit at the expense of image quality. In this work, we use a U-Net architecture to reduce artifacts from sparse-view CCTs, predicting fully sampled reconstructions from sparse-view ones. We evaluate the hemorrhage detectability in the predicted CCTs with a hemorrhage classification convolutional neural network, trained on fully sampled CCTs to detect and classify different sub-types of hemorrhages. Our results suggest that the automated classification and detection accuracy of hemorrhages in sparse-view CCTs can be improved substantially by the U-Net. This demonstrates the feasibility of rapid automated hemorrhage detection on low-dose CT data to assist radiologists in routine clinical practice.
Optimizing Convolutional Neural Networks for Chronic Obstructive Pulmonary Disease Detection in Clinical Computed Tomography Imaging
Mar 13, 2023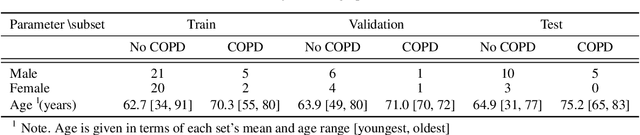
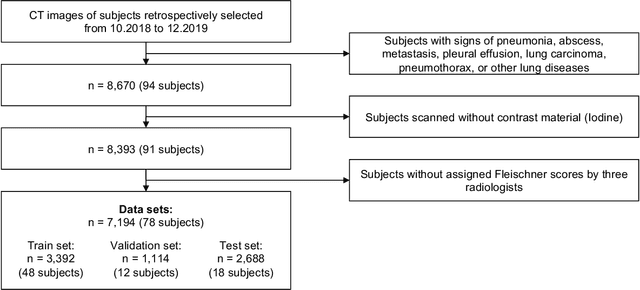
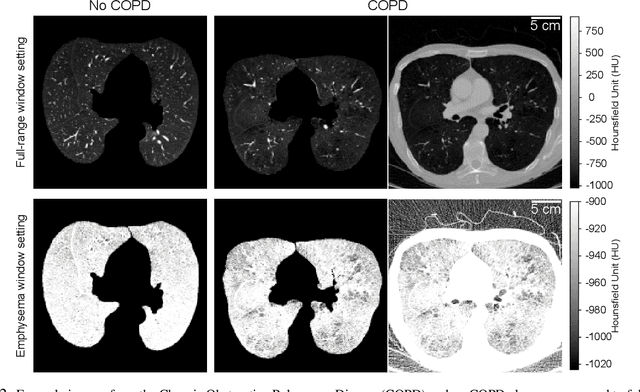
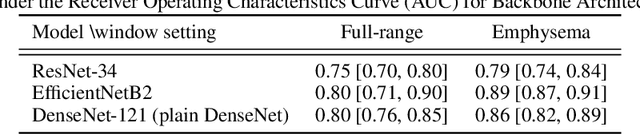
Abstract:Chronic Obstructive Pulmonary Disease (COPD) is a leading cause of death worldwide, yet early detection and treatment can prevent the progression of the disease. In contrast to the conventional method of detecting COPD with spirometry tests, X-ray Computed Tomography (CT) scans of the chest provide a measure of morphological changes in the lung. It has been shown that automated detection of COPD can be performed with deep learning models. However, the potential of incorporating optimal window setting selection, typically carried out by clinicians during examination of CT scans for COPD, is generally overlooked in deep learning approaches. We aim to optimize the binary classification of COPD with densely connected convolutional neural networks (DenseNets) through implementation of manual and automated Window-Setting Optimization (WSO) steps. Our dataset consisted of 78 CT scans from the Klinikum rechts der Isar research hospital. Repeated inference on the test set showed that without WSO, the plain DenseNet resulted in a mean slice-level AUC of 0.80$\pm$0.05. With input images manually adjusted to the emphysema window setting, the plain DenseNet model predicted COPD with a mean AUC of 0.86$\pm$0.04. By automating the WSO through addition of a customized layer to the DenseNet, an optimal window setting in the proximity of the emphysema window setting was learned and a mean AUC of 0.82$\pm$0.04 was achieved. Detection of COPD with DenseNet models was optimized by WSO of CT data to the emphysema window setting range, demonstrating the importance of implementing optimal window setting selection in the deep learning pipeline.
Per-Pixel Lung Thickness and Lung Capacity Estimation on Chest X-Rays using Convolutional Neural Networks
Oct 27, 2021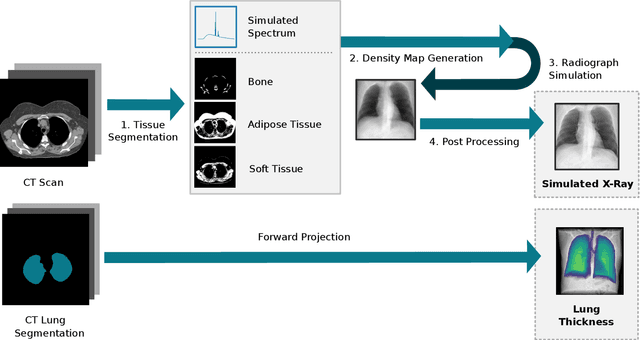
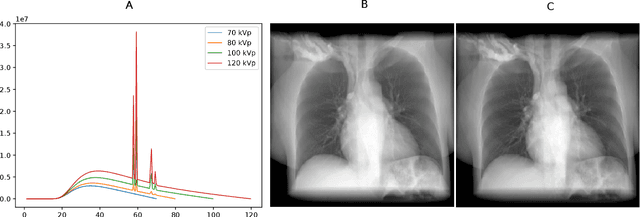
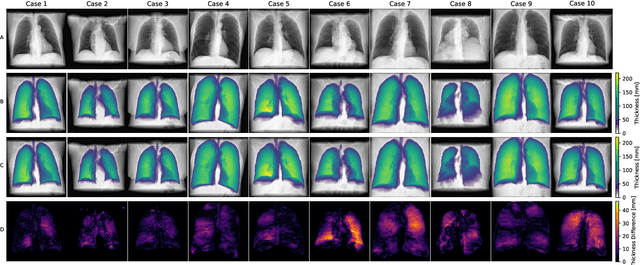
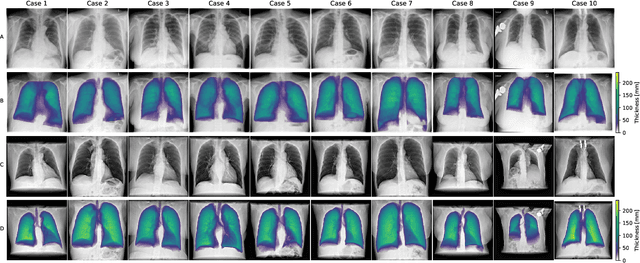
Abstract:Estimating the lung depth on x-ray images could provide both an accurate opportunistic lung volume estimation during clinical routine and improve image contrast in modern structural chest imaging techniques like x-ray dark-field imaging. We present a method based on a convolutional neural network that allows a per-pixel lung thickness estimation and subsequent total lung capacity estimation. The network was trained and validated using 5250 simulated radiographs generated from 525 real CT scans. Furthermore, we are able to infer the model trained with simulation data on real radiographs. For 35 patients, quantitative and qualitative evaluation was performed on standard clinical radiographs. The ground-truth for each patient's total lung volume was defined based on the patients' corresponding CT scan. The mean-absolute error between the estimated lung volume on the 35 real radiographs and groundtruth volume was 0.73 liter. Additionally, we predicted the lung thicknesses on a synthetic dataset of 131 radiographs, where the mean-absolute error was 0.27 liter. The results show, that it is possible to transfer the knowledge obtained in a simulation model to real x-ray images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge