Florian Schaff
Chair of Biomedical Physics, Department of Physics, School of Natural Sciences, Technical University of Munich, Germany, Munich Institute of Biomedical Engineering, Technical University of Munich, Germany
Uncertainty-guided Generation of Dark-field Radiographs
Jan 22, 2026Abstract:X-ray dark-field radiography provides complementary diagnostic information to conventional attenuation imaging by visualizing microstructural tissue changes through small-angle scattering. However, the limited availability of such data poses challenges for developing robust deep learning models. In this work, we present the first framework for generating dark-field images directly from standard attenuation chest X-rays using an Uncertainty-Guided Progressive Generative Adversarial Network. The model incorporates both aleatoric and epistemic uncertainty to improve interpretability and reliability. Experiments demonstrate high structural fidelity of the generated images, with consistent improvement of quantitative metrics across stages. Furthermore, out-of-distribution evaluation confirms that the proposed model generalizes well. Our results indicate that uncertainty-guided generative modeling enables realistic dark-field image synthesis and provides a reliable foundation for future clinical applications.
Beam Geometry and Input Dimensionality: Impact on Sparse-Sampling Artifact Correction for Clinical CT with U-Nets
Aug 25, 2025Abstract:This study aims to investigate the effect of various beam geometries and dimensions of input data on the sparse-sampling streak artifact correction task with U-Nets for clinical CT scans as a means of incorporating the volumetric context into artifact reduction tasks to improve model performance. A total of 22 subjects were retrospectively selected (01.2016-12.2018) from the Technical University of Munich's research hospital, TUM Klinikum rechts der Isar. Sparsely-sampled CT volumes were simulated with the Astra toolbox for parallel, fan, and cone beam geometries. 2048 views were taken as full-view scans. 2D and 3D U-Nets were trained and validated on 14, and tested on 8 subjects, respectively. For the dimensionality study, in addition to the 512x512 2D CT images, the CT scans were further pre-processed to generate a so-called '2.5D', and 3D data: Each CT volume was divided into 64x64x64 voxel blocks. The 3D data refers to individual 64-voxel blocks. An axial, coronal, and sagittal cut through the center of each block resulted in three 64x64 2D patches that were rearranged as a single 64x64x3 image, proposed as 2.5D data. Model performance was assessed with the mean squared error (MSE) and structural similarity index measure (SSIM). For all geometries, the 2D U-Net trained on axial 2D slices results in the best MSE and SSIM values, outperforming the 2.5D and 3D input data dimensions.
Improving Automated Hemorrhage Detection in Sparse-view Computed Tomography via Deep Convolutional Neural Network based Artifact Reduction
Mar 16, 2023Abstract:Intracranial hemorrhage poses a serious health problem requiring rapid and often intensive medical treatment. For diagnosis, a Cranial Computed Tomography (CCT) scan is usually performed. However, the increased health risk caused by radiation is a concern. The most important strategy to reduce this potential risk is to keep the radiation dose as low as possible and consistent with the diagnostic task. Sparse-view CT can be an effective strategy to reduce dose by reducing the total number of views acquired, albeit at the expense of image quality. In this work, we use a U-Net architecture to reduce artifacts from sparse-view CCTs, predicting fully sampled reconstructions from sparse-view ones. We evaluate the hemorrhage detectability in the predicted CCTs with a hemorrhage classification convolutional neural network, trained on fully sampled CCTs to detect and classify different sub-types of hemorrhages. Our results suggest that the automated classification and detection accuracy of hemorrhages in sparse-view CCTs can be improved substantially by the U-Net. This demonstrates the feasibility of rapid automated hemorrhage detection on low-dose CT data to assist radiologists in routine clinical practice.
Optimizing Convolutional Neural Networks for Chronic Obstructive Pulmonary Disease Detection in Clinical Computed Tomography Imaging
Mar 13, 2023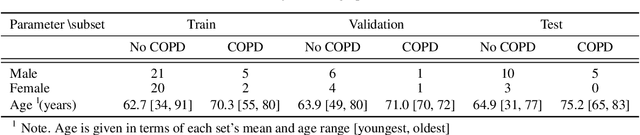
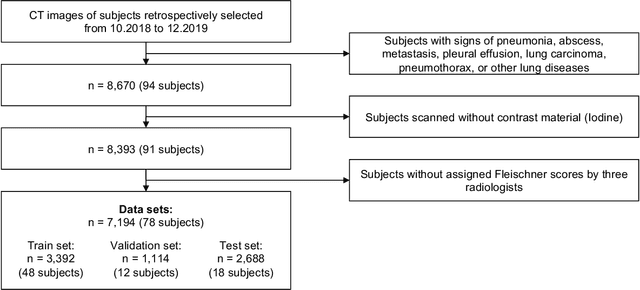
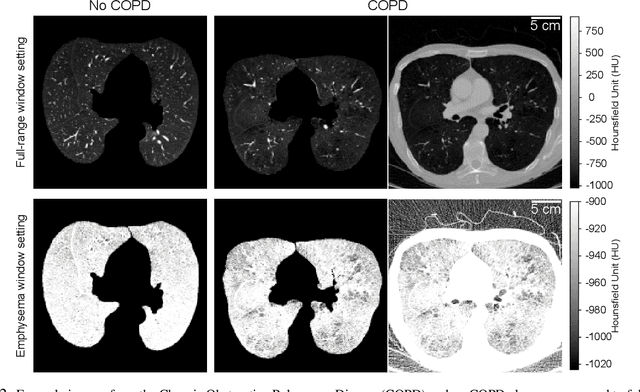
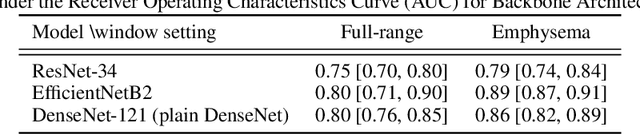
Abstract:Chronic Obstructive Pulmonary Disease (COPD) is a leading cause of death worldwide, yet early detection and treatment can prevent the progression of the disease. In contrast to the conventional method of detecting COPD with spirometry tests, X-ray Computed Tomography (CT) scans of the chest provide a measure of morphological changes in the lung. It has been shown that automated detection of COPD can be performed with deep learning models. However, the potential of incorporating optimal window setting selection, typically carried out by clinicians during examination of CT scans for COPD, is generally overlooked in deep learning approaches. We aim to optimize the binary classification of COPD with densely connected convolutional neural networks (DenseNets) through implementation of manual and automated Window-Setting Optimization (WSO) steps. Our dataset consisted of 78 CT scans from the Klinikum rechts der Isar research hospital. Repeated inference on the test set showed that without WSO, the plain DenseNet resulted in a mean slice-level AUC of 0.80$\pm$0.05. With input images manually adjusted to the emphysema window setting, the plain DenseNet model predicted COPD with a mean AUC of 0.86$\pm$0.04. By automating the WSO through addition of a customized layer to the DenseNet, an optimal window setting in the proximity of the emphysema window setting was learned and a mean AUC of 0.82$\pm$0.04 was achieved. Detection of COPD with DenseNet models was optimized by WSO of CT data to the emphysema window setting range, demonstrating the importance of implementing optimal window setting selection in the deep learning pipeline.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge