Mahdieh Kazemimoghadam
Leveraging Global Binary Masks for Structure Segmentation in Medical Images
May 13, 2022



Abstract:Deep learning (DL) models for medical image segmentation are highly influenced by intensity variations of input images and lack generalization due to primarily utilizing pixels' intensity information for inference. Acquiring sufficient training data is another challenge limiting models' applications. We proposed to leverage the consistency of organs' anatomical shape and position information in medical images. We introduced a framework leveraging recurring anatomical patterns through global binary masks for organ segmentation. Two scenarios were studied.1) Global binary masks were the only model's (i.e. U-Net) input, forcing exclusively encoding organs' position and shape information for segmentation/localization.2) Global binary masks were incorporated as an additional channel functioning as position/shape clues to mitigate training data scarcity. Two datasets of the brain and heart CT images with their ground-truth were split into (26:10:10) and (12:3:5) for training, validation, and test respectively. Training exclusively on global binary masks led to Dice scores of 0.77(0.06) and 0.85(0.04), with the average Euclidian distance of 3.12(1.43)mm and 2.5(0.93)mm relative to the center of mass of the ground truth for the brain and heart structures respectively. The outcomes indicate that a surprising degree of position and shape information is encoded through global binary masks. Incorporating global binary masks led to significantly higher accuracy relative to the model trained on only CT images in small subsets of training data; the performance improved by 4.3-125.3% and 1.3-48.1% for 1-8 training cases of the brain and heart datasets respectively. The findings imply the advantages of utilizing global binary masks for building generalizable models and to compensate for training data scarcity.
An Activity Recognition Framework for Continuous Monitoring of Non-Steady-State Locomotion of Individuals with Parkinson's Disease
Oct 08, 2021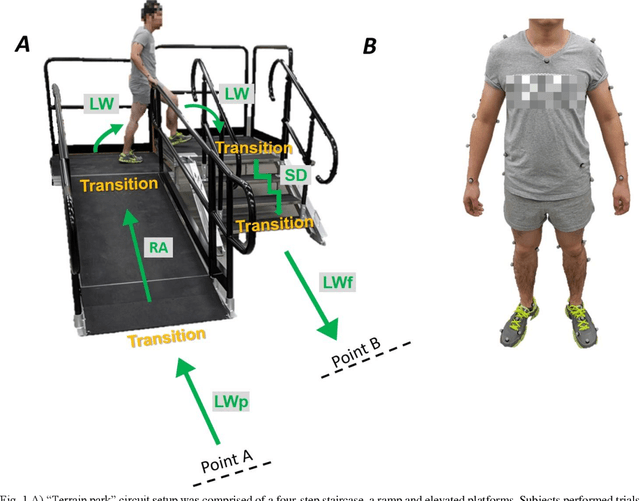
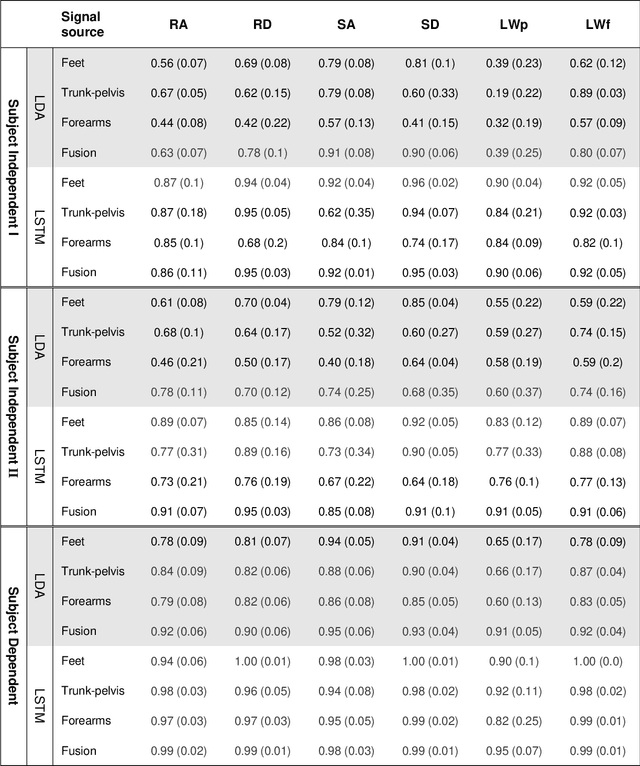
Abstract:Fundamental knowledge in activity recognition of individuals with motor disorders such as Parkinson's disease (PD) has been primarily limited to detection of steady-state/static tasks (sitting, standing, walking). To date, identification of non-steady-state locomotion on uneven terrains (stairs, ramps) has not received much attention. Furthermore, previous research has mainly relied on data from a large number of body locations which could adversely affect user convenience and system performance. Here, individuals with mild stages of PD and healthy subjects performed non-steady-state circuit trials comprising stairs, ramp, and changes of direction. An offline analysis using a linear discriminant analysis (LDA) classifier and a Long-Short Term Memory (LSTM) neural network was performed for task recognition. The performance of accelerographic and gyroscopic information from varied lower/upper-body segments were tested across a set of user-independent and user-dependent training paradigms. Comparing the F1 score of a given signal across classifiers showed improved performance using LSTM compared to LDA. Using LSTM, even a subset of information (e.g., feet data) in subject-independent training appeared to provide F1 score > 0.8. However, employing LDA was shown to be at the expense of being limited to using a subject-dependent training and/or biomechanical data from multiple body locations. The findings could inform a number of applications in the field of healthcare monitoring and developing advanced lower-limb assistive devices by providing insights into classification schemes capable of handling non-steady-state and unstructured locomotion in individuals with mild Parkinson's disease.
Saliency-Guided Deep Learning Network for Automatic Tumor Bed Volume Delineation in Post-operative Breast Irradiation
May 06, 2021



Abstract:Efficient, reliable and reproducible target volume delineation is a key step in the effective planning of breast radiotherapy. However, post-operative breast target delineation is challenging as the contrast between the tumor bed volume (TBV) and normal breast tissue is relatively low in CT images. In this study, we propose to mimic the marker-guidance procedure in manual target delineation. We developed a saliency-based deep learning segmentation (SDL-Seg) algorithm for accurate TBV segmentation in post-operative breast irradiation. The SDL-Seg algorithm incorporates saliency information in the form of markers' location cues into a U-Net model. The design forces the model to encode the location-related features, which underscores regions with high saliency levels and suppresses low saliency regions. The saliency maps were generated by identifying markers on CT images. Markers' locations were then converted to probability maps using a distance-transformation coupled with a Gaussian filter. Subsequently, the CT images and the corresponding saliency maps formed a multi-channel input for the SDL-Seg network. Our in-house dataset was comprised of 145 prone CT images from 29 post-operative breast cancer patients, who received 5-fraction partial breast irradiation (PBI) regimen on GammaPod. The performance of the proposed method was compared against basic U-Net. Our model achieved mean (standard deviation) of 76.4 %, 6.76 mm, and 1.9 mm for DSC, HD95, and ASD respectively on the test set with computation time of below 11 seconds per one CT volume. SDL-Seg showed superior performance relative to basic U-Net for all the evaluation metrics while preserving low computation cost. The findings demonstrate that SDL-Seg is a promising approach for improving the efficiency and accuracy of the on-line treatment planning procedure of PBI, such as GammaPod based PBI.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge