Lukas Alexander Wilhelm Gemein
Deep Riemannian Networks for EEG Decoding
Dec 21, 2022Abstract:State-of-the-art performance in electroencephalography (EEG) decoding tasks is currently often achieved with either Deep-Learning or Riemannian-Geometry-based decoders. Recently, there is growing interest in Deep Riemannian Networks (DRNs) possibly combining the advantages of both previous classes of methods. However, there are still a range of topics where additional insight is needed to pave the way for a more widespread application of DRNs in EEG. These include architecture design questions such as network size and end-to-end ability as well as model training questions. How these factors affect model performance has not been explored. Additionally, it is not clear how the data within these networks is transformed, and whether this would correlate with traditional EEG decoding. Our study aims to lay the groundwork in the area of these topics through the analysis of DRNs for EEG with a wide range of hyperparameters. Networks were tested on two public EEG datasets and compared with state-of-the-art ConvNets. Here we propose end-to-end EEG SPDNet (EE(G)-SPDNet), and we show that this wide, end-to-end DRN can outperform the ConvNets, and in doing so use physiologically plausible frequency regions. We also show that the end-to-end approach learns more complex filters than traditional band-pass filters targeting the classical alpha, beta, and gamma frequency bands of the EEG, and that performance can benefit from channel specific filtering approaches. Additionally, architectural analysis revealed areas for further improvement due to the possible loss of Riemannian specific information throughout the network. Our study thus shows how to design and train DRNs to infer task-related information from the raw EEG without the need of handcrafted filterbanks and highlights the potential of end-to-end DRNs such as EE(G)-SPDNet for high-performance EEG decoding.
Machine-Learning-Based Diagnostics of EEG Pathology
Feb 11, 2020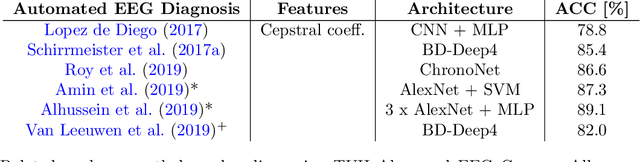
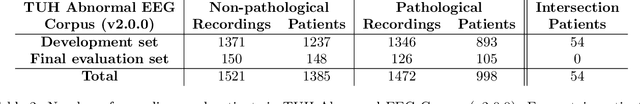
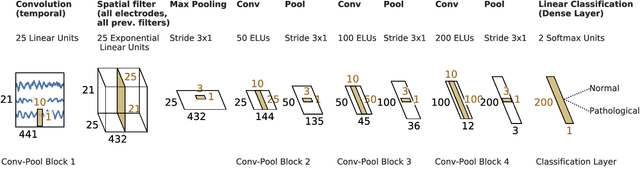
Abstract:Machine learning (ML) methods have the potential to automate clinical EEG analysis. They can be categorized into feature-based (with handcrafted features), and end-to-end approaches (with learned features). Previous studies on EEG pathology decoding have typically analyzed a limited number of features, decoders, or both. For a I) more elaborate feature-based EEG analysis, and II) in-depth comparisons of both approaches, here we first develop a comprehensive feature-based framework, and then compare this framework to state-of-the-art end-to-end methods. To this aim, we apply the proposed feature-based framework and deep neural networks including an EEG-optimized temporal convolutional network (TCN) to the task of pathological versus non-pathological EEG classification. For a robust comparison, we chose the Temple University Hospital (TUH) Abnormal EEG Corpus (v2.0.0), which contains approximately 3000 EEG recordings. The results demonstrate that the proposed feature-based decoding framework can achieve accuracies on the same level as state-of-the-art deep neural networks. We find accuracies across both approaches in an astonishingly narrow range from 81--86\%. Moreover, visualizations and analyses indicated that both approaches used similar aspects of the data, e.g., delta and theta band power at temporal electrode locations. We argue that the accuracies of current binary EEG pathology decoders could saturate near 90\% due to the imperfect inter-rater agreement of the clinical labels, and that such decoders are already clinically useful, such as in areas where clinical EEG experts are rare. We make the proposed feature-based framework available open source and thus offer a new tool for EEG machine learning research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge