Laura Mora Ballestar
QU-BraTS: MICCAI BraTS 2020 Challenge on Quantifying Uncertainty in Brain Tumor Segmentation -- Analysis of Ranking Metrics and Benchmarking Results
Dec 19, 2021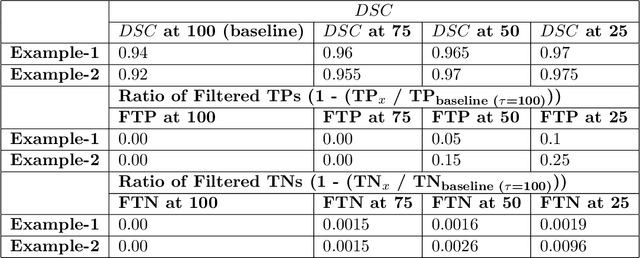
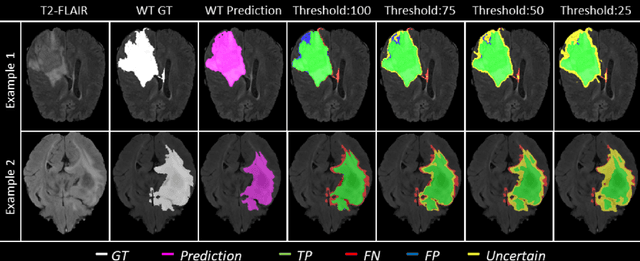
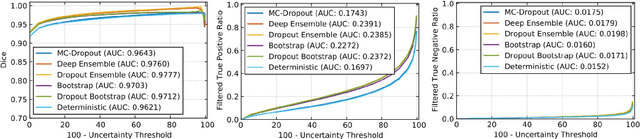
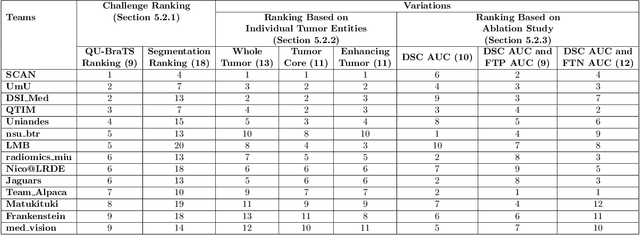
Abstract:Deep learning (DL) models have provided the state-of-the-art performance in a wide variety of medical imaging benchmarking challenges, including the Brain Tumor Segmentation (BraTS) challenges. However, the task of focal pathology multi-compartment segmentation (e.g., tumor and lesion sub-regions) is particularly challenging, and potential errors hinder the translation of DL models into clinical workflows. Quantifying the reliability of DL model predictions in the form of uncertainties, could enable clinical review of the most uncertain regions, thereby building trust and paving the way towards clinical translation. Recently, a number of uncertainty estimation methods have been introduced for DL medical image segmentation tasks. Developing metrics to evaluate and compare the performance of uncertainty measures will assist the end-user in making more informed decisions. In this study, we explore and evaluate a metric developed during the BraTS 2019-2020 task on uncertainty quantification (QU-BraTS), and designed to assess and rank uncertainty estimates for brain tumor multi-compartment segmentation. This metric (1) rewards uncertainty estimates that produce high confidence in correct assertions, and those that assign low confidence levels at incorrect assertions, and (2) penalizes uncertainty measures that lead to a higher percentages of under-confident correct assertions. We further benchmark the segmentation uncertainties generated by 14 independent participating teams of QU-BraTS 2020, all of which also participated in the main BraTS segmentation task. Overall, our findings confirm the importance and complementary value that uncertainty estimates provide to segmentation algorithms, and hence highlight the need for uncertainty quantification in medical image analyses. Our evaluation code is made publicly available at https://github.com/RagMeh11/QU-BraTS.
MRI brain tumor segmentation and uncertainty estimation using 3D-UNet architectures
Dec 30, 2020
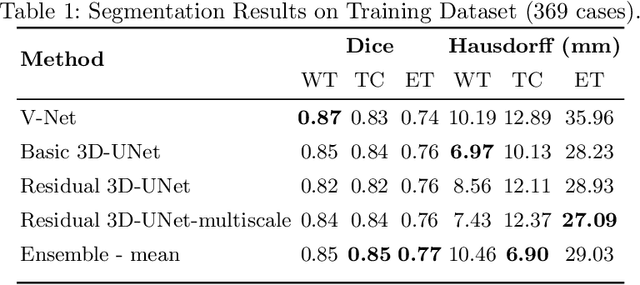
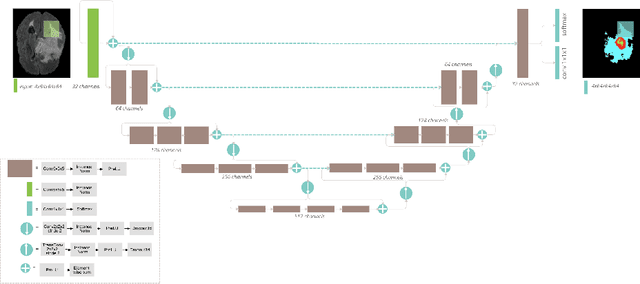
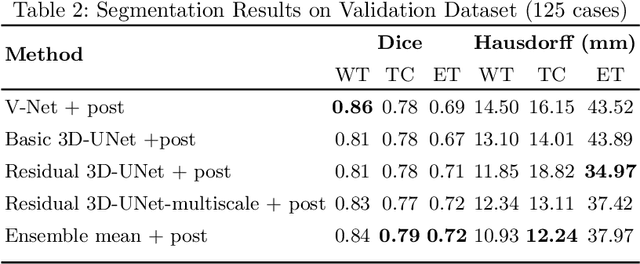
Abstract:Automation of brain tumor segmentation in 3D magnetic resonance images (MRIs) is key to assess the diagnostic and treatment of the disease. In recent years, convolutional neural networks (CNNs) have shown improved results in the task. However, high memory consumption is still a problem in 3D-CNNs. Moreover, most methods do not include uncertainty information, which is especially critical in medical diagnosis. This work studies 3D encoder-decoder architectures trained with patch-based techniques to reduce memory consumption and decrease the effect of unbalanced data. The different trained models are then used to create an ensemble that leverages the properties of each model, thus increasing the performance. We also introduce voxel-wise uncertainty information, both epistemic and aleatoric using test-time dropout (TTD) and data-augmentation (TTA) respectively. In addition, a hybrid approach is proposed that helps increase the accuracy of the segmentation. The model and uncertainty estimation measurements proposed in this work have been used in the BraTS'20 Challenge for task 1 and 3 regarding tumor segmentation and uncertainty estimation.
Brain Tumor Segmentation using 3D-CNNs with Uncertainty Estimation
Sep 24, 2020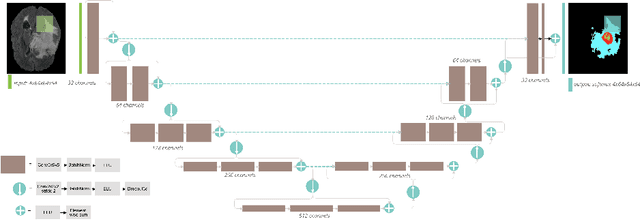
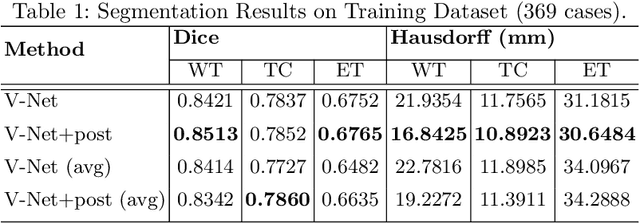
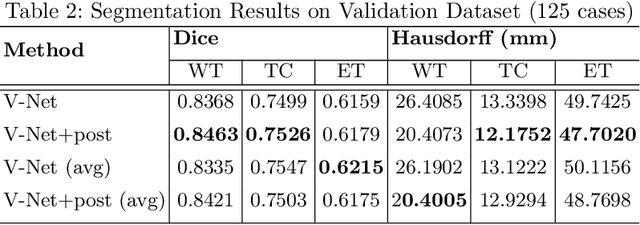
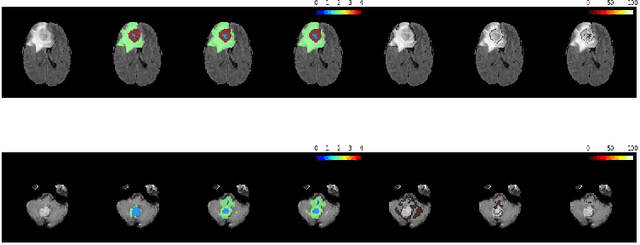
Abstract:Automation of brain tumors in 3D magnetic resonance images (MRIs) is key to assess the diagnostic and treatment of the disease. In recent years, convolutional neural networks (CNNs) have shown improved results in the task. However, high memory consumption is still a problem in 3D-CNNs. Moreover, most methods do not include uncertainty information, which is specially critical in medical diagnosis. This work proposes a 3D encoder-decoder architecture, based on V-Net \cite{vnet} which is trained with patching techniques to reduce memory consumption and decrease the effect of unbalanced data. We also introduce voxel-wise uncertainty, both epistemic and aleatoric using test-time dropout and data-augmentation respectively. Uncertainty maps can provide extra information to expert neurologists, useful for detecting when the model is not confident on the provided segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge