Khaled Younis
Persistence Image from 3D Medical Image: Superpixel and Optimized Gaussian Coefficient
Aug 15, 2024Abstract:Topological data analysis (TDA) uncovers crucial properties of objects in medical imaging. Methods based on persistent homology have demonstrated their advantages in capturing topological features that traditional deep learning methods cannot detect in both radiology and pathology. However, previous research primarily focused on 2D image analysis, neglecting the comprehensive 3D context. In this paper, we propose an innovative 3D TDA approach that incorporates the concept of superpixels to transform 3D medical image features into point cloud data. By Utilizing Optimized Gaussian Coefficient, the proposed 3D TDA method, for the first time, efficiently generate holistic Persistence Images for 3D volumetric data. Our 3D TDA method exhibits superior performance on the MedMNist3D dataset when compared to other traditional methods, showcasing its potential effectiveness in modeling 3D persistent homology-based topological analysis when it comes to classification tasks. The source code is publicly available at https://github.com/hrlblab/TopologicalDataAnalysis3D.
RAISE -- Radiology AI Safety, an End-to-end lifecycle approach
Nov 24, 2023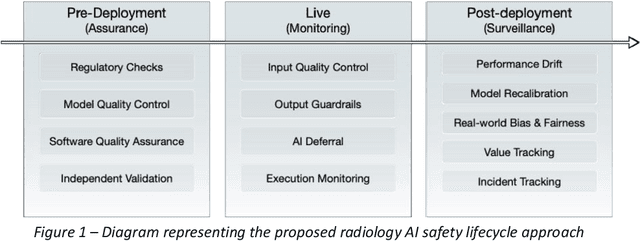
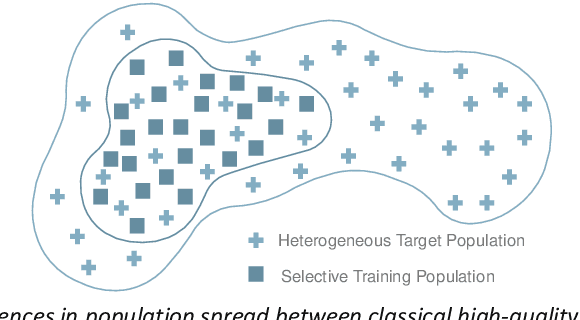
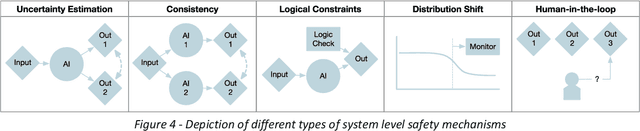
Abstract:The integration of AI into radiology introduces opportunities for improved clinical care provision and efficiency but it demands a meticulous approach to mitigate potential risks as with any other new technology. Beginning with rigorous pre-deployment evaluation and validation, the focus should be on ensuring models meet the highest standards of safety, effectiveness and efficacy for their intended applications. Input and output guardrails implemented during production usage act as an additional layer of protection, identifying and addressing individual failures as they occur. Continuous post-deployment monitoring allows for tracking population-level performance (data drift), fairness, and value delivery over time. Scheduling reviews of post-deployment model performance and educating radiologists about new algorithmic-driven findings is critical for AI to be effective in clinical practice. Recognizing that no single AI solution can provide absolute assurance even when limited to its intended use, the synergistic application of quality assurance at multiple levels - regulatory, clinical, technical, and ethical - is emphasized. Collaborative efforts between stakeholders spanning healthcare systems, industry, academia, and government are imperative to address the multifaceted challenges involved. Trust in AI is an earned privilege, contingent on a broad set of goals, among them transparently demonstrating that the AI adheres to the same rigorous safety, effectiveness and efficacy standards as other established medical technologies. By doing so, developers can instil confidence among providers and patients alike, enabling the responsible scaling of AI and the realization of its potential benefits. The roadmap presented herein aims to expedite the achievement of deployable, reliable, and safe AI in radiology.
Current State of Community-Driven Radiological AI Deployment in Medical Imaging
Dec 29, 2022



Abstract:Artificial Intelligence (AI) has become commonplace to solve routine everyday tasks. Because of the exponential growth in medical imaging data volume and complexity, the workload on radiologists is steadily increasing. We project that the gap between the number of imaging exams and the number of expert radiologist readers required to cover this increase will continue to expand, consequently introducing a demand for AI-based tools that improve the efficiency with which radiologists can comfortably interpret these exams. AI has been shown to improve efficiency in medical-image generation, processing, and interpretation, and a variety of such AI models have been developed across research labs worldwide. However, very few of these, if any, find their way into routine clinical use, a discrepancy that reflects the divide between AI research and successful AI translation. To address the barrier to clinical deployment, we have formed MONAI Consortium, an open-source community which is building standards for AI deployment in healthcare institutions, and developing tools and infrastructure to facilitate their implementation. This report represents several years of weekly discussions and hands-on problem solving experience by groups of industry experts and clinicians in the MONAI Consortium. We identify barriers between AI-model development in research labs and subsequent clinical deployment and propose solutions. Our report provides guidance on processes which take an imaging AI model from development to clinical implementation in a healthcare institution. We discuss various AI integration points in a clinical Radiology workflow. We also present a taxonomy of Radiology AI use-cases. Through this report, we intend to educate the stakeholders in healthcare and AI (AI researchers, radiologists, imaging informaticists, and regulators) about cross-disciplinary challenges and possible solutions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge