Kaouther Mouheb
MedSapiens: Taking a Pose to Rethink Medical Imaging Landmark Detection
Nov 06, 2025Abstract:This paper does not introduce a novel architecture; instead, it revisits a fundamental yet overlooked baseline: adapting human-centric foundation models for anatomical landmark detection in medical imaging. While landmark detection has traditionally relied on domain-specific models, the emergence of large-scale pre-trained vision models presents new opportunities. In this study, we investigate the adaptation of Sapiens, a human-centric foundation model designed for pose estimation, to medical imaging through multi-dataset pretraining, establishing a new state of the art across multiple datasets. Our proposed model, MedSapiens, demonstrates that human-centric foundation models, inherently optimized for spatial pose localization, provide strong priors for anatomical landmark detection, yet this potential has remained largely untapped. We benchmark MedSapiens against existing state-of-the-art models, achieving up to 5.26% improvement over generalist models and up to 21.81% improvement over specialist models in the average success detection rate (SDR). To further assess MedSapiens adaptability to novel downstream tasks with few annotations, we evaluate its performance in limited-data settings, achieving 2.69% improvement over the few-shot state of the art in SDR. Code and model weights are available at https://github.com/xmed-lab/MedSapiens .
Evaluating the Fairness of Neural Collapse in Medical Image Classification
Jul 08, 2024Abstract:Deep learning has achieved impressive performance across various medical imaging tasks. However, its inherent bias against specific groups hinders its clinical applicability in equitable healthcare systems. A recently discovered phenomenon, Neural Collapse (NC), has shown potential in improving the generalization of state-of-the-art deep learning models. Nonetheless, its implications on bias in medical imaging remain unexplored. Our study investigates deep learning fairness through the lens of NC. We analyze the training dynamics of models as they approach NC when training using biased datasets, and examine the subsequent impact on test performance, specifically focusing on label bias. We find that biased training initially results in different NC configurations across subgroups, before converging to a final NC solution by memorizing all data samples. Through extensive experiments on three medical imaging datasets -- PAPILA, HAM10000, and CheXpert -- we find that in biased settings, NC can lead to a significant drop in F1 score across all subgroups. Our code is available at https://gitlab.com/radiology/neuro/neural-collapse-fairness
Large Intestine 3D Shape Refinement Using Point Diffusion Models for Digital Phantom Generation
Sep 15, 2023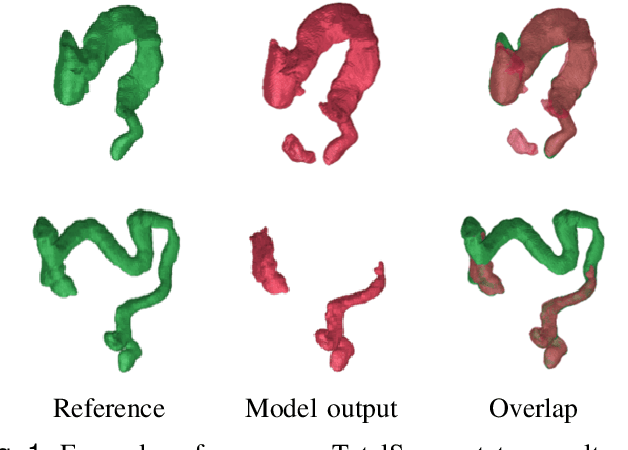
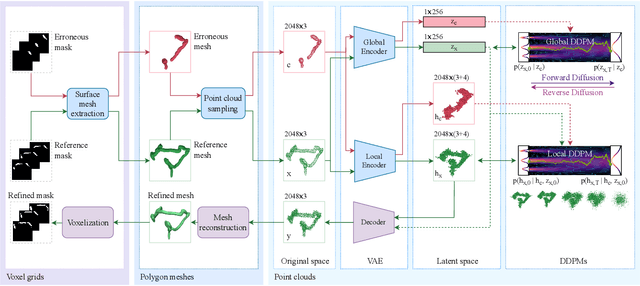
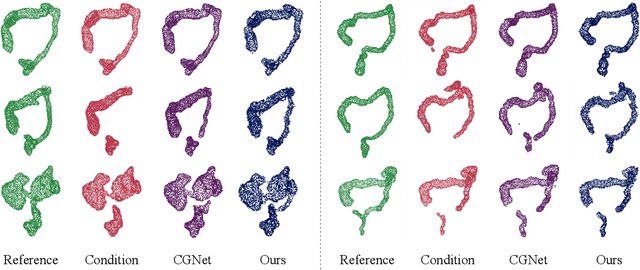
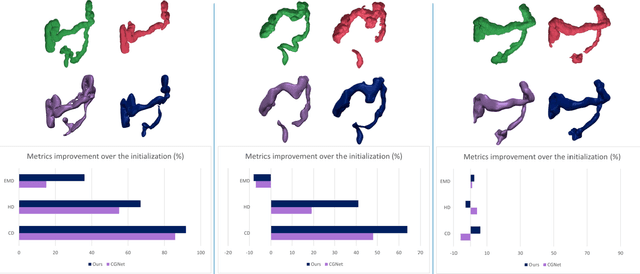
Abstract:Accurate 3D modeling of human organs plays a crucial role in building computational phantoms for virtual imaging trials. However, generating anatomically plausible reconstructions of organ surfaces from computed tomography scans remains challenging for many structures in the human body. This challenge is particularly evident when dealing with the large intestine. In this study, we leverage recent advancements in geometric deep learning and denoising diffusion probabilistic models to refine the segmentation results of the large intestine. We begin by representing the organ as point clouds sampled from the surface of the 3D segmentation mask. Subsequently, we employ a hierarchical variational autoencoder to obtain global and local latent representations of the organ's shape. We train two conditional denoising diffusion models in the hierarchical latent space to perform shape refinement. To further enhance our method, we incorporate a state-of-the-art surface reconstruction model, allowing us to generate smooth meshes from the obtained complete point clouds. Experimental results demonstrate the effectiveness of our approach in capturing both the global distribution of the organ's shape and its fine details. Our complete refinement pipeline demonstrates remarkable enhancements in surface representation compared to the initial segmentation, reducing the Chamfer distance by 70%, the Hausdorff distance by 32%, and the Earth Mover's distance by 6%. By combining geometric deep learning, denoising diffusion models, and advanced surface reconstruction techniques, our proposed method offers a promising solution for accurately modeling the large intestine's surface and can easily be extended to other anatomical structures.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge