Kanika Dheman
.Project Based Learning Center, ETH Zürich, Switzerland, .Multi-Scale Robotics Lab, ETH Zürich, Switzerland
i-CardiAx: Wearable IoT-Driven System for Early Sepsis Detection Through Long-Term Vital Sign Monitoring
Jul 31, 2024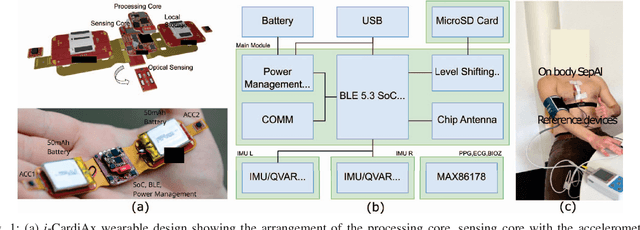

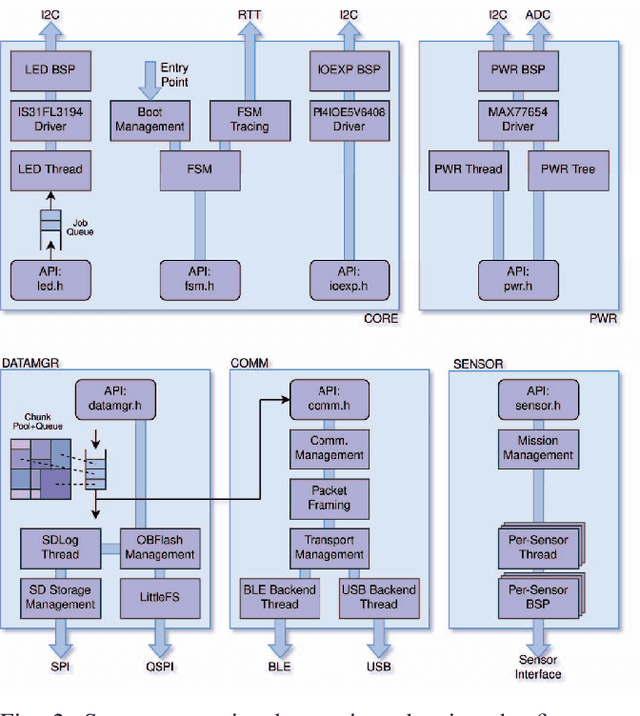

Abstract:Sepsis is a significant cause of early mortality, high healthcare costs, and disability-adjusted life years. Digital interventions like continuous cardiac monitoring can help detect early warning signs and facilitate effective interventions. This paper introduces i-CardiAx, a wearable sensor utilizing low-power high-sensitivity accelerometers to measure vital signs crucial for cardiovascular health: heart rate (HR), blood pressure (BP), and respiratory rate (RR). Data collected from 10 healthy subjects using the i-CardiAx chest patch were used to develop and evaluate lightweight vital sign measurement algorithms. The algorithms demonstrated high performance: RR (-0.11 $\pm$ 0.77 breaths\min), HR (0.82 $\pm$ 2.85 beats\min), and systolic BP (-0.08 $\pm$ 6.245 mmHg). These algorithms are embedded in an ARM Cortex-M33 processor with Bluetooth Low Energy (BLE) support, achieving inference times of 4.2 ms for HR and RR, and 8.5 ms for BP. Additionally, a multi-channel quantized Temporal Convolutional Neural (TCN) Network, trained on the open-source HiRID dataset, was developed to detect sepsis onset using digitally acquired vital signs from i-CardiAx. The quantized TCN, deployed on i-CardiAx, predicted sepsis with a median time of 8.2 hours and an energy per inference of 1.29 mJ. The i-CardiAx wearable boasts a sleep power of 0.152 mW and an average power consumption of 0.77 mW, enabling a 100 mAh battery to last approximately two weeks (432 hours) with continuous monitoring of HR, BP, and RR at 30 measurements per hour and running inference every 30 minutes. In conclusion, i-CardiAx offers an energy-efficient, high-sensitivity method for long-term cardiovascular monitoring, providing predictive alerts for sepsis and other life-threatening events.
Earable and Wrist-worn Setup for Accurate Step Counting Utilizing Body-Area Electrostatic Sensing
Jul 08, 2024Abstract:Step-counting has been widely implemented in wrist-worn devices and is accepted by end users as a quantitative indicator of everyday exercise. However, existing counting approach (mostly on wrist-worn setup) lacks robustness and thus introduces inaccuracy issues in certain scenarios like brief intermittent walking bouts and random arm motions or static arm status while walking (no clear correlation of motion pattern between arm and leg). This paper proposes a low-power step-counting solution utilizing the body area electric field acquired by a novel electrostatic sensing unit, consuming only 87.3 $\mu$W of power, hoping to strengthen the robustness of current dominant solution. We designed two wearable devices for on-the-wrist and in-the-ear deployment and collected body-area electric field-derived motion signals from ten volunteers. Four walking scenarios are considered: in the parking lot/shopping center with/without pushing the shopping trolley. The step-counting accuracy from the prototypes shows better accuracy than the commercial wrist-worn devices (e.g.,96% of the wrist- and ear-worn prototype vs. 66% of the Fitbit when walking in the shopping center while pushing a shopping trolley). We finally discussed the potential and limitations of sensing body-area electric fields for wrist-worn and ear-worn step-counting and beyond.
Investigation of mmWave Radar Technology For Non-contact Vital Sign Monitoring
Sep 15, 2023Abstract:Non-contact vital sign monitoring has many advantages over conventional methods in being comfortable, unobtrusive and without any risk of spreading infection. The use of millimeter-wave (mmWave) radars is one of the most promising approaches that enable contact-less monitoring of vital signs. Novel low-power implementations of this technology promise to enable vital sign sensing in embedded, battery-operated devices. The nature of these new low-power sensors exacerbates the challenges of accurate and robust vital sign monitoring and especially the problem of heart-rate tracking. This work focuses on the investigation and characterization of three Frequency Modulated Continuous Wave (FMCW) low-power radars with different carrier frequencies of 24 GHz, 60 GHz and 120 GHz. The evaluation platforms were first tested on phantom models that emulated human bodies to accurately evaluate the baseline noise, error in range estimation, and error in displacement estimation. Additionally, the systems were also used to collect data from three human subjects to gauge the feasibility of identifying heartbeat peaks and breathing peaks with simple and lightweight algorithms that could potentially run in low-power embedded processors. The investigation revealed that the 24 GHz radar has the highest baseline noise level, 0.04mm at 0{\deg} angle of incidence, and an error in range estimation of 3.45 +- 1.88 cm at a distance of 60 cm. At the same distance, the 60 GHz and the 120 GHz radar system shows the least noise level, 0.0lmm at 0{\deg} angle of incidence, and error in range estimation 0.64 +- 0.01 cm and 0.04 +- 0.0 cm respectively. Additionally, tests on humans showed that all three radar systems were able to identify heart and breathing activity but the 120 GHz radar system outperformed the other two.
Non-invasive urinary bladder volume estimation with artefact-suppressed bio-impedance measurements
Mar 24, 2023



Abstract:Urine output is a vital parameter to gauge kidney health. Current monitoring methods include manually written records, invasive urinary catheterization or ultrasound measurements performed by highly skilled personnel. Catheterization bears high risks of infection while intermittent ultrasound measures and manual recording are time consuming and might miss early signs of kidney malfunction. Bioimpedance (BI) measurements may serve as a non-invasive alternative for measuring urine volume in vivo. However, limited robustness have prevented its clinical translation. Here, a deep learning-based algorithm is presented that processes the local BI of the lower abdomen and suppresses artefacts to measure the bladder volume quantitatively, non-invasively and without the continuous need for additional personnel. A tetrapolar BI wearable system called ANUVIS was used to collect continuous bladder volume data from three healthy subjects to demonstrate feasibility of operation, while clinical gold standards of urodynamic (n=6) and uroflowmetry tests (n=8) provided the ground truth. Optimized location for electrode placement and a model for the change in BI with changing bladder volume is deduced. The average error for full bladder volume estimation and for residual volume estimation was -29 +/-87.6 ml, thus, comparable to commercial portable ultrasound devices (Bland Altman analysis showed a bias of -5.2 ml with LoA between 119.7 ml to -130.1 ml), while providing the additional benefit of hands-free, non-invasive, and continuous bladder volume estimation. The combination of the wearable BI sensor node and the presented algorithm provides an attractive alternative to current standard of care with potential benefits in providing insights into kidney function.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge