Kalliopi Dalakleidi
A comprehensive interpretable machine learning framework for Mild Cognitive Impairment and Alzheimer's disease diagnosis
Dec 12, 2024



Abstract:An interpretable machine learning (ML) framework is introduced to enhance the diagnosis of Mild Cognitive Impairment (MCI) and Alzheimer's disease (AD) by ensuring robustness of the ML models' interpretations. The dataset used comprises volumetric measurements from brain MRI and genetic data from healthy individuals and patients with MCI/AD, obtained through the Alzheimer's Disease Neuroimaging Initiative. The existing class imbalance is addressed by an ensemble learning approach, while various attribution-based and counterfactual-based interpretability methods are leveraged towards producing diverse explanations related to the pathophysiology of MCI/AD. A unification method combining SHAP with counterfactual explanations assesses the interpretability techniques' robustness. The best performing model yielded 87.5% balanced accuracy and 90.8% F1-score. The attribution-based interpretability methods highlighted significant volumetric and genetic features related to MCI/AD risk. The unification method provided useful insights regarding those features' necessity and sufficiency, further showcasing their significance in MCI/AD diagnosis.
Stratification of carotid atheromatous plaque using interpretable deep learning methods on B-mode ultrasound images
Feb 04, 2022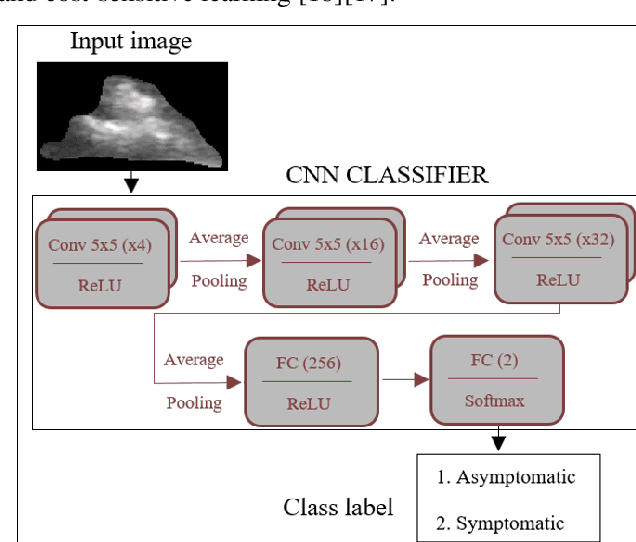
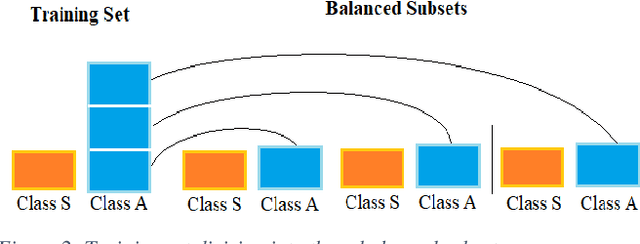
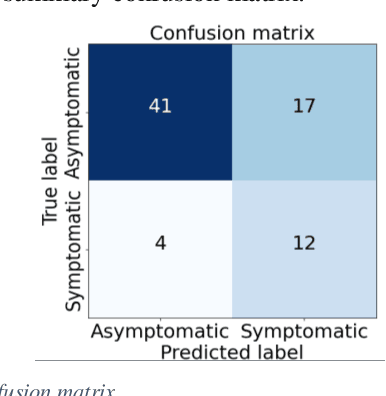
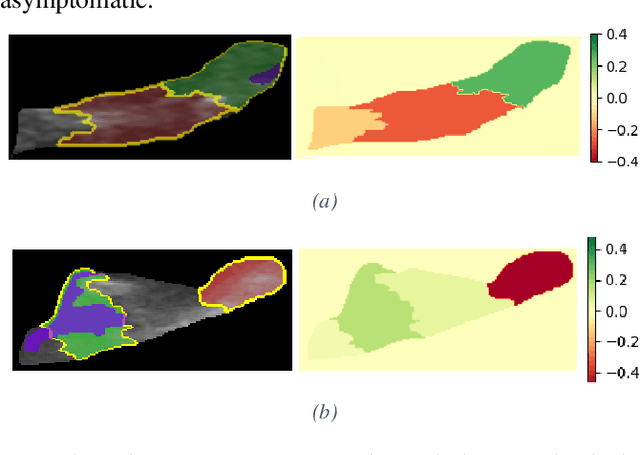
Abstract:Carotid atherosclerosis is the major cause of ischemic stroke resulting in significant rates of mortality and disability annually. Early diagnosis of such cases is of great importance, since it enables clinicians to apply a more effective treatment strategy. This paper introduces an interpretable classification approach of carotid ultrasound images for the risk assessment and stratification of patients with carotid atheromatous plaque. To address the highly imbalanced distribution of patients between the symptomatic and asymptomatic classes (16 vs 58, respectively), an ensemble learning scheme based on a sub-sampling approach was applied along with a two-phase, cost-sensitive strategy of learning, that uses the original and a resampled data set. Convolutional Neural Networks (CNNs) were utilized for building the primary models of the ensemble. A six-layer deep CNN was used to automatically extract features from the images, followed by a classification stage of two fully connected layers. The obtained results (Area Under the ROC Curve (AUC): 73%, sensitivity: 75%, specificity: 70%) indicate that the proposed approach achieved acceptable discrimination performance. Finally, interpretability methods were applied on the model's predictions in order to reveal insights on the model's decision process as well as to enable the identification of novel image biomarkers for the stratification of patients with carotid atheromatous plaque.Clinical Relevance-The integration of interpretability methods with deep learning strategies can facilitate the identification of novel ultrasound image biomarkers for the stratification of patients with carotid atheromatous plaque.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge