Konstantina S Nikita
Machine Learning Method for Functional Assessment of Retinal Models
Feb 05, 2022
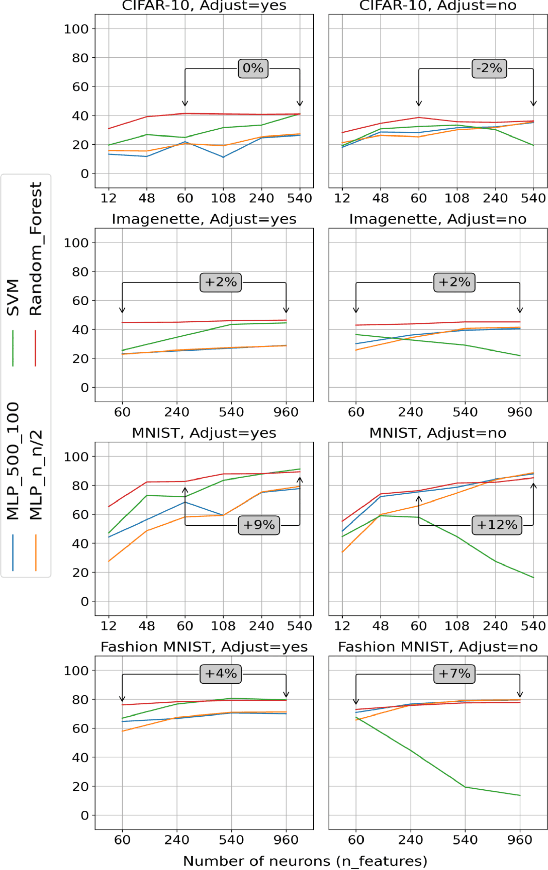
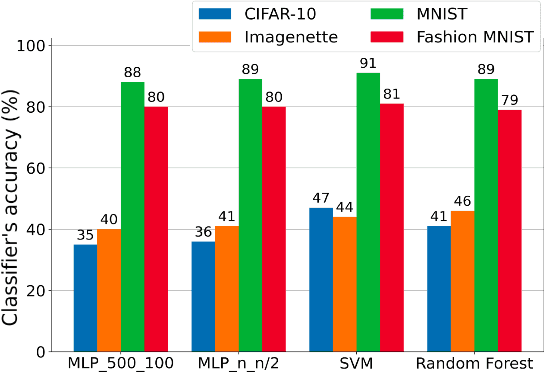
Abstract:Challenges in the field of retinal prostheses motivate the development of retinal models to accurately simulate Retinal Ganglion Cells (RGCs) responses. The goal of retinal prostheses is to enable blind individuals to solve complex, reallife visual tasks. In this paper, we introduce the functional assessment (FA) of retinal models, which describes the concept of evaluating the performance of retinal models on visual understanding tasks. We present a machine learning method for FA: we feed traditional machine learning classifiers with RGC responses generated by retinal models, to solve object and digit recognition tasks (CIFAR-10, MNIST, Fashion MNIST, Imagenette). We examined critical FA aspects, including how the performance of FA depends on the task, how to optimally feed RGC responses to the classifiers and how the number of output neurons correlates with the model's accuracy. To increase the number of output neurons, we manipulated input images - by splitting and then feeding them to the retinal model and we found that image splitting does not significantly improve the model's accuracy. We also show that differences in the structure of datasets result in largely divergent performance of the retinal model (MNIST and Fashion MNIST exceeded 80% accuracy, while CIFAR-10 and Imagenette achieved ~40%). Furthermore, retinal models which perform better in standard evaluation, i.e. more accurately predict RGC response, perform better in FA as well. However, unlike standard evaluation, FA results can be straightforwardly interpreted in the context of comparing the quality of visual perception.
Stratification of carotid atheromatous plaque using interpretable deep learning methods on B-mode ultrasound images
Feb 04, 2022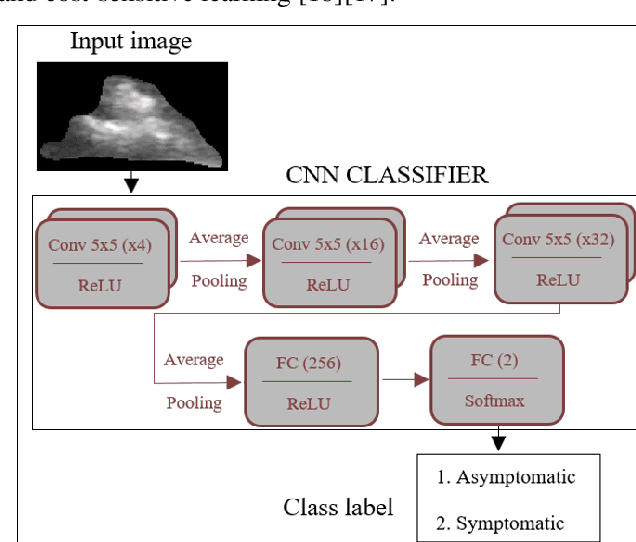
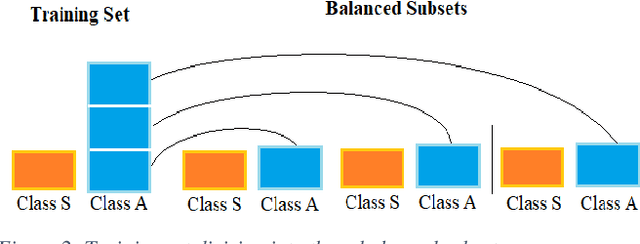
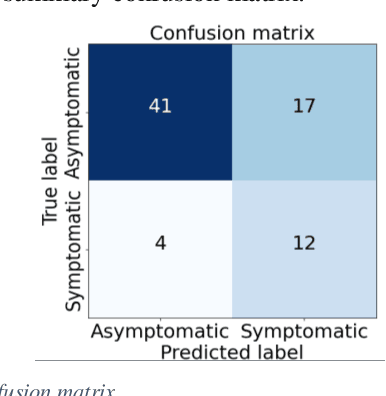
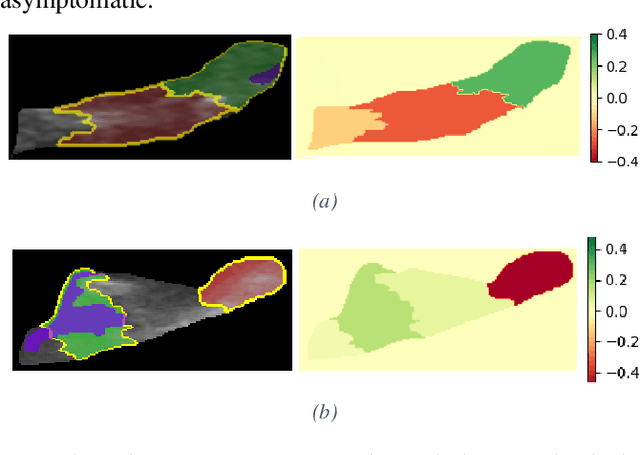
Abstract:Carotid atherosclerosis is the major cause of ischemic stroke resulting in significant rates of mortality and disability annually. Early diagnosis of such cases is of great importance, since it enables clinicians to apply a more effective treatment strategy. This paper introduces an interpretable classification approach of carotid ultrasound images for the risk assessment and stratification of patients with carotid atheromatous plaque. To address the highly imbalanced distribution of patients between the symptomatic and asymptomatic classes (16 vs 58, respectively), an ensemble learning scheme based on a sub-sampling approach was applied along with a two-phase, cost-sensitive strategy of learning, that uses the original and a resampled data set. Convolutional Neural Networks (CNNs) were utilized for building the primary models of the ensemble. A six-layer deep CNN was used to automatically extract features from the images, followed by a classification stage of two fully connected layers. The obtained results (Area Under the ROC Curve (AUC): 73%, sensitivity: 75%, specificity: 70%) indicate that the proposed approach achieved acceptable discrimination performance. Finally, interpretability methods were applied on the model's predictions in order to reveal insights on the model's decision process as well as to enable the identification of novel image biomarkers for the stratification of patients with carotid atheromatous plaque.Clinical Relevance-The integration of interpretability methods with deep learning strategies can facilitate the identification of novel ultrasound image biomarkers for the stratification of patients with carotid atheromatous plaque.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge