Joris Roels
Cost-efficient segmentation of electron microscopy images using active learning
Nov 13, 2019



Abstract:Over the last decade, electron microscopy has improved up to a point that generating high quality gigavoxel sized datasets only requires a few hours. Automated image analysis, particularly image segmentation, however, has not evolved at the same pace. Even though state-of-the-art methods such as U-Net and DeepLab have improved segmentation performance substantially, the required amount of labels remains too expensive. Active learning is the subfield in machine learning that aims to mitigate this burden by selecting the samples that require labeling in a smart way. Many techniques have been proposed, particularly for image classification, to increase the steepness of learning curves. In this work, we extend these techniques to deep CNN based image segmentation. Our experiments on three different electron microscopy datasets show that active learning can improve segmentation quality by 10 to 15% in terms of Jaccard score compared to standard randomized sampling.
Bayesian Deconvolution of Scanning Electron Microscopy Images Using Point-spread Function Estimation and Non-local Regularization
Oct 23, 2018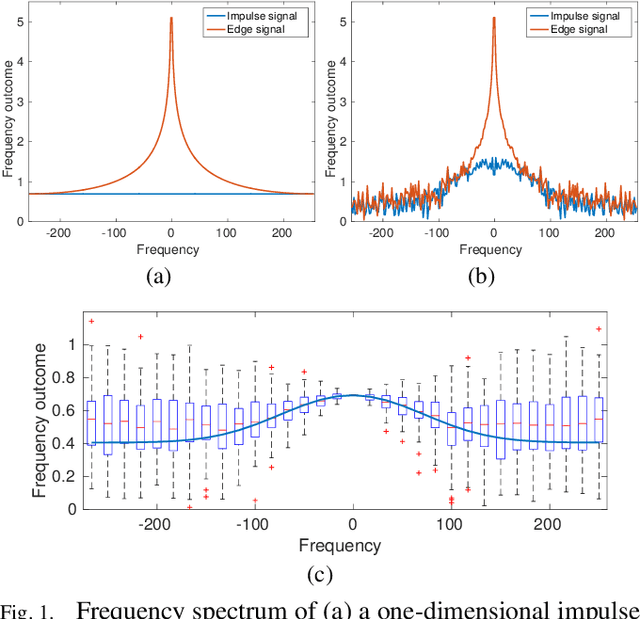
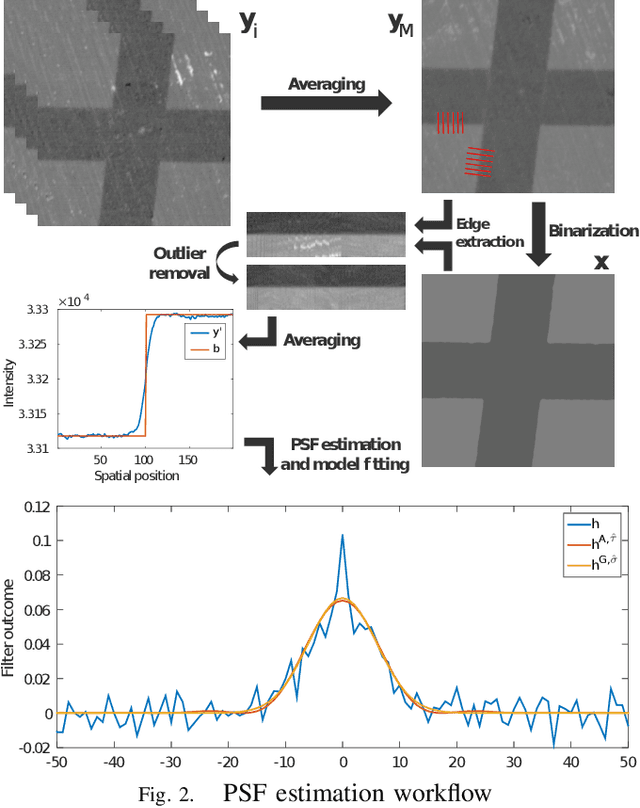
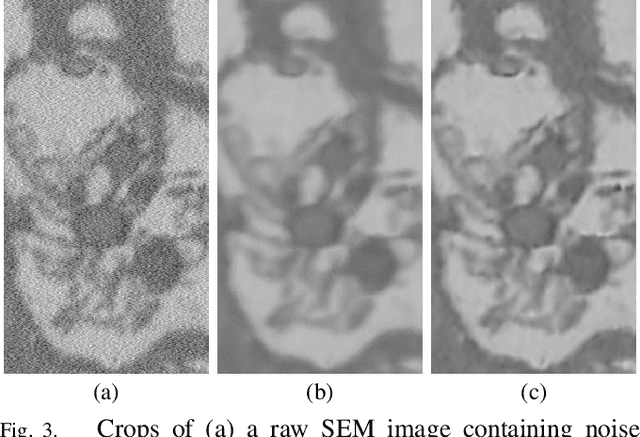
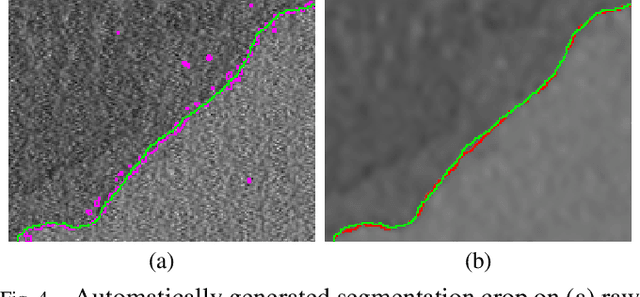
Abstract:Microscopy is one of the most essential imaging techniques in life sciences. High-quality images are required in order to solve (potentially life-saving) biomedical research problems. Many microscopy techniques do not achieve sufficient resolution for these purposes, being limited by physical diffraction and hardware deficiencies. Electron microscopy addresses optical diffraction by measuring emitted or transmitted electrons instead of photons, yielding nanometer resolution. Despite pushing back the diffraction limit, blur should still be taken into account because of practical hardware imperfections and remaining electron diffraction. Deconvolution algorithms can remove some of the blur in post-processing but they depend on knowledge of the point-spread function (PSF) and should accurately regularize noise. Any errors in the estimated PSF or noise model will reduce their effectiveness. This paper proposes a new procedure to estimate the lateral component of the point spread function of a 3D scanning electron microscope more accurately. We also propose a Bayesian maximum a posteriori deconvolution algorithm with a non-local image prior which employs this PSF estimate and previously developed noise statistics. We demonstrate visual quality improvements and show that applying our method improves the quality of subsequent segmentation steps.
Convolutional Neural Network Pruning to Accelerate Membrane Segmentation in Electron Microscopy
Oct 23, 2018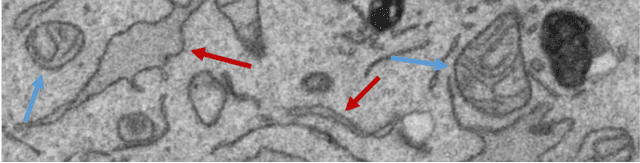
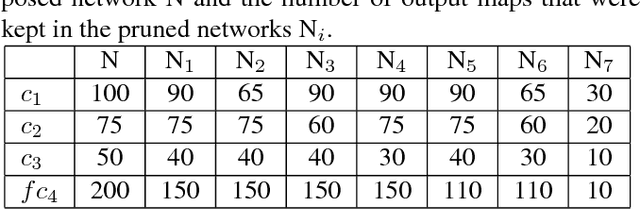
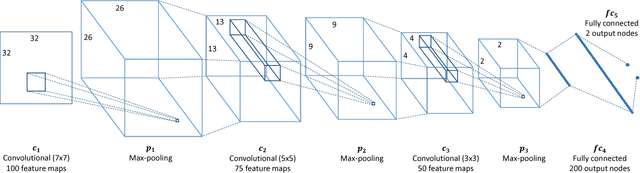
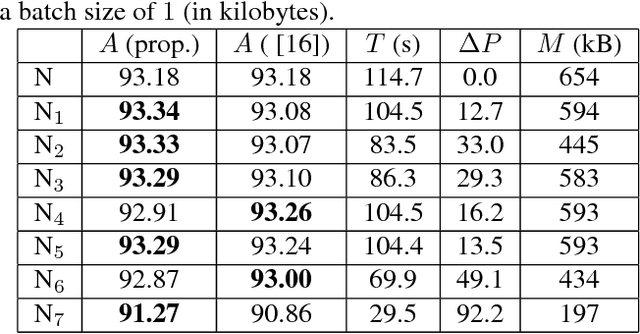
Abstract:Biological membranes are one of the most basic structures and regions of interest in cell biology. In the study of membranes, segment extraction is a well-known and difficult problem because of impeding noise, directional and thickness variability, etc. Recent advances in electron microscopy membrane segmentation are able to cope with such difficulties by training convolutional neural networks. However, because of the massive amount of features that have to be extracted while propagating forward, the practical usability diminishes, even with state-of-the-art GPU's. A significant part of these network features typically contains redundancy through correlation and sparsity. In this work, we propose a pruning method for convolutional neural networks that ensures the training loss increase is minimized. We show that the pruned networks, after retraining, are more efficient in terms of time and memory, without significantly affecting the network accuracy. This way, we manage to obtain real-time membrane segmentation performance, for our specific electron microscopy setup.
Domain Adaptive Segmentation in Volume Electron Microscopy Imaging
Oct 23, 2018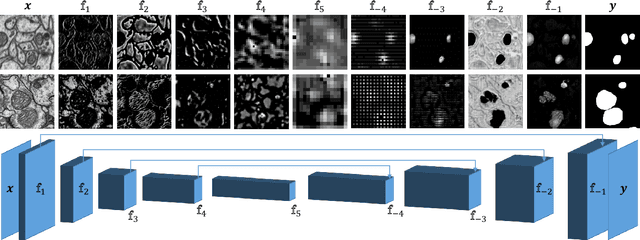
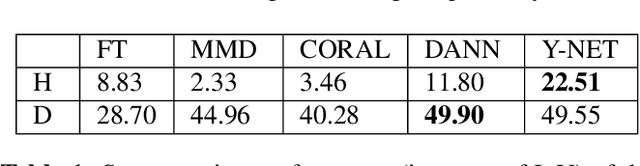
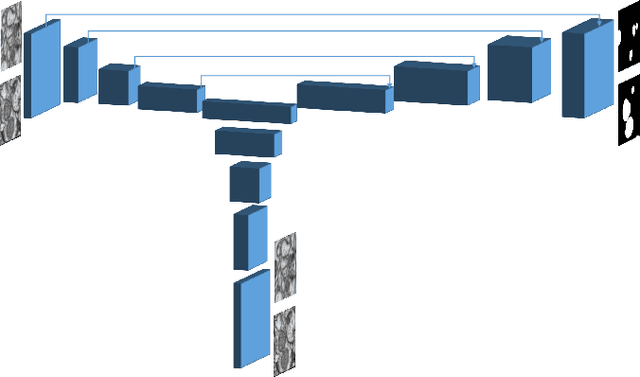
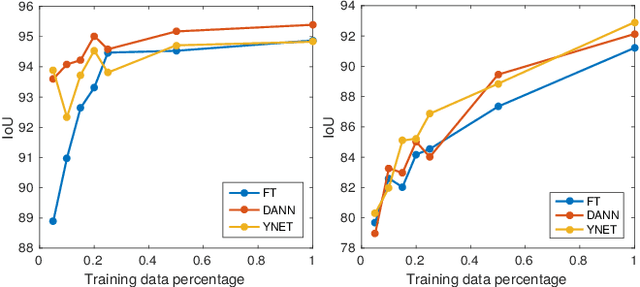
Abstract:In the last years, automated segmentation has become a necessary tool for volume electron microscopy (EM) imaging. So far, the best performing techniques have been largely based on fully supervised encoder-decoder CNNs, requiring a substantial amount of annotated images. Domain Adaptation (DA) aims to alleviate the annotation burden by 'adapting' the networks trained on existing groundtruth data (source domain) to work on a different (target) domain with as little additional annotation as possible. Most DA research is focused on the classification task, whereas volume EM segmentation remains rather unexplored. In this work, we extend recently proposed classification DA techniques to an encoder-decoder layout and propose a novel method that adds a reconstruction decoder to the classical encoder-decoder segmentation in order to align source and target encoder features. The method has been validated on the task of segmenting mitochondria in EM volumes. We have performed DA from brain EM images to HeLa cells and from isotropic FIB/SEM volumes to anisotropic TEM volumes. In all cases, the proposed method has outperformed the extended classification DA techniques and the finetuning baseline. An implementation of our work can be found on https://github.com/JorisRoels/domain-adaptive-segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge