Julian Hennies
Leveraging Domain Knowledge to improve EM image segmentation with Lifted Multicuts
May 25, 2019



Abstract:The throughput of electron microscopes has increased significantly in recent years, enabling detailed analysis of cell morphology and ultrastructure. Analysis of neural circuits at single-synapse resolution remains the flagship target of this technique, but applications to cell and developmental biology are also starting to emerge at scale. The amount of data acquired in such studies makes manual instance segmentation, a fundamental step in many analysis pipelines, impossible. While automatic segmentation approaches have improved significantly thanks to the adoption of convolutional neural networks, their accuracy still lags behind human annotations and requires additional manual proof-reading. A major hindrance to further improvements is the limited field of view of the segmentation networks preventing them from exploiting the expected cell morphology or other prior biological knowledge which humans use to inform their segmentation decisions. In this contribution, we show how such domain-specific information can be leveraged by expressing it as long-range interactions in a graph partitioning problem known as the lifted multicut problem. Using this formulation, we demonstrate significant improvement in segmentation accuracy for three challenging EM segmentation problems from neuroscience and cell biology.
Domain Adaptive Segmentation in Volume Electron Microscopy Imaging
Oct 23, 2018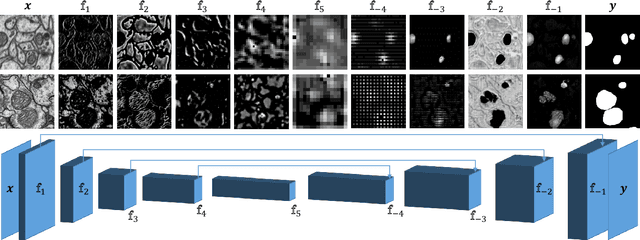
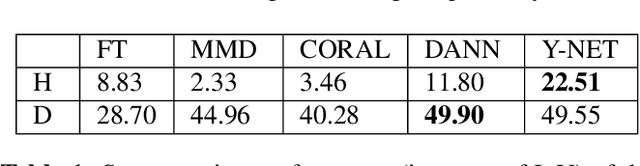
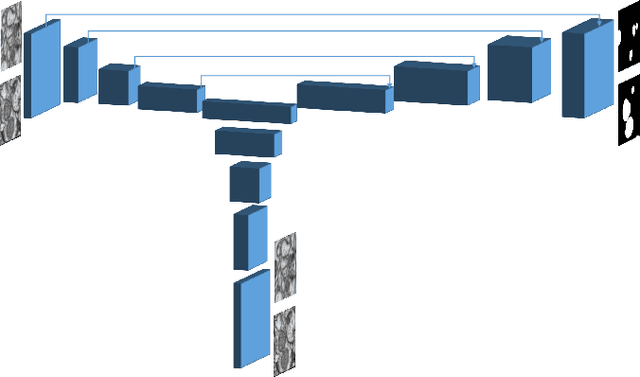
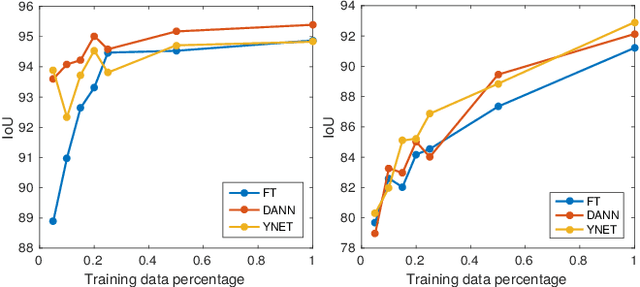
Abstract:In the last years, automated segmentation has become a necessary tool for volume electron microscopy (EM) imaging. So far, the best performing techniques have been largely based on fully supervised encoder-decoder CNNs, requiring a substantial amount of annotated images. Domain Adaptation (DA) aims to alleviate the annotation burden by 'adapting' the networks trained on existing groundtruth data (source domain) to work on a different (target) domain with as little additional annotation as possible. Most DA research is focused on the classification task, whereas volume EM segmentation remains rather unexplored. In this work, we extend recently proposed classification DA techniques to an encoder-decoder layout and propose a novel method that adds a reconstruction decoder to the classical encoder-decoder segmentation in order to align source and target encoder features. The method has been validated on the task of segmenting mitochondria in EM volumes. We have performed DA from brain EM images to HeLa cells and from isotropic FIB/SEM volumes to anisotropic TEM volumes. In all cases, the proposed method has outperformed the extended classification DA techniques and the finetuning baseline. An implementation of our work can be found on https://github.com/JorisRoels/domain-adaptive-segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge