Joost Daemen
GReAT: leveraging geometric artery data to improve wall shear stress assessment
Aug 26, 2025Abstract:Leveraging big data for patient care is promising in many medical fields such as cardiovascular health. For example, hemodynamic biomarkers like wall shear stress could be assessed from patient-specific medical images via machine learning algorithms, bypassing the need for time-intensive computational fluid simulation. However, it is extremely challenging to amass large-enough datasets to effectively train such models. We could address this data scarcity by means of self-supervised pre-training and foundations models given large datasets of geometric artery models. In the context of coronary arteries, leveraging learned representations to improve hemodynamic biomarker assessment has not yet been well studied. In this work, we address this gap by investigating whether a large dataset (8449 shapes) consisting of geometric models of 3D blood vessels can benefit wall shear stress assessment in coronary artery models from a small-scale clinical trial (49 patients). We create a self-supervised target for the 3D blood vessels by computing the heat kernel signature, a quantity obtained via Laplacian eigenvectors, which captures the very essence of the shapes. We show how geometric representations learned from this datasets can boost segmentation of coronary arteries into regions of low, mid and high (time-averaged) wall shear stress even when trained on limited data.
Classifier-guided registration of coronary CT angiography and intravascular ultrasound
Dec 22, 2024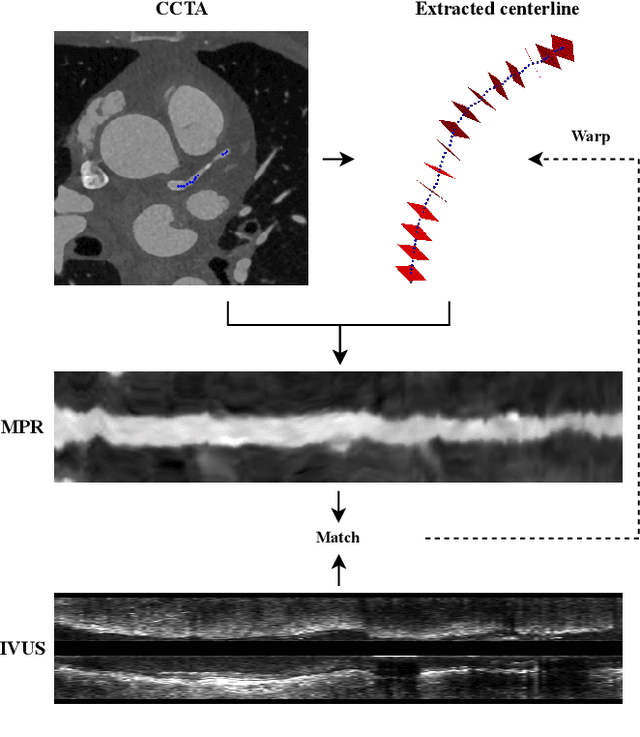
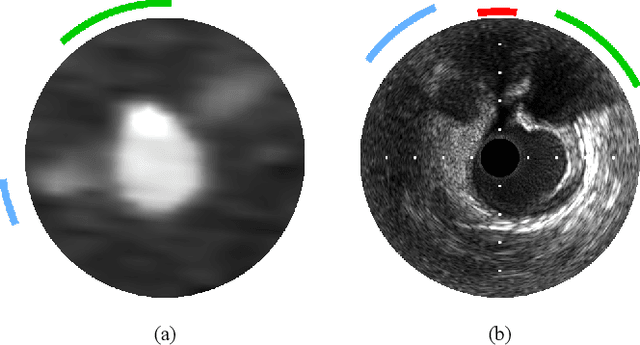
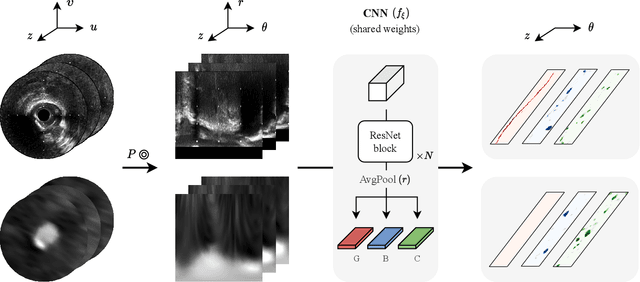
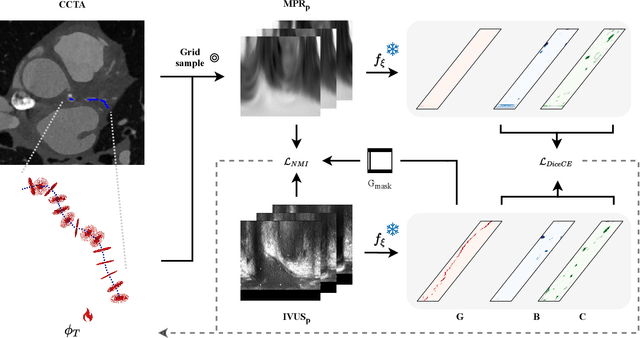
Abstract:Coronary CT angiography (CCTA) and intravascular ultrasound (IVUS) provide complementary information for coronary artery disease assessment, making their registration valuable for comprehensive analysis. However, existing registration methods require manual interaction or extensive segmentations, limiting their practical application. In this work, we present a fully automatic framework for CCTA-IVUS registration using deep learning-based feature detection and a differentiable image registration module. Our approach leverages a convolutional neural network trained to identify key anatomical features from polar-transformed multiplanar reformatted CCTA or IVUS data. These detected anatomical featuers subsequently guide a differentiable registration module to optimize transformation parameters of an automatically extracted coronary artery centerline. The method does not require landmark selection or segmentations as input, while accounting for the presence of IVUS guidewire artifacts. Evaluated on 48 clinical cases with reference CCTA centerlines corresponding to IVUS pullback, our method achieved successful registration in 83.3\% of cases, with a median centerline overlap F$_1$-score of 0.982 and median cosine similarities of 0.940 and 0.944 for cross-sectional plane orientation. Our results demonstrate that automatically detected anatomical features can be leveraged for accurate registration. The fully automatic nature of the approach represents a significant step toward streamlined multimodal coronary analysis, potentially facilitating large-scale studies of coronary plaque characteristics across modalities.
Segmentation of Anatomical Layers and Artifacts in Intravascular Polarization Sensitive Optical Coherence Tomography Using Attending Physician and Boundary Cardinality Lost Terms
May 11, 2021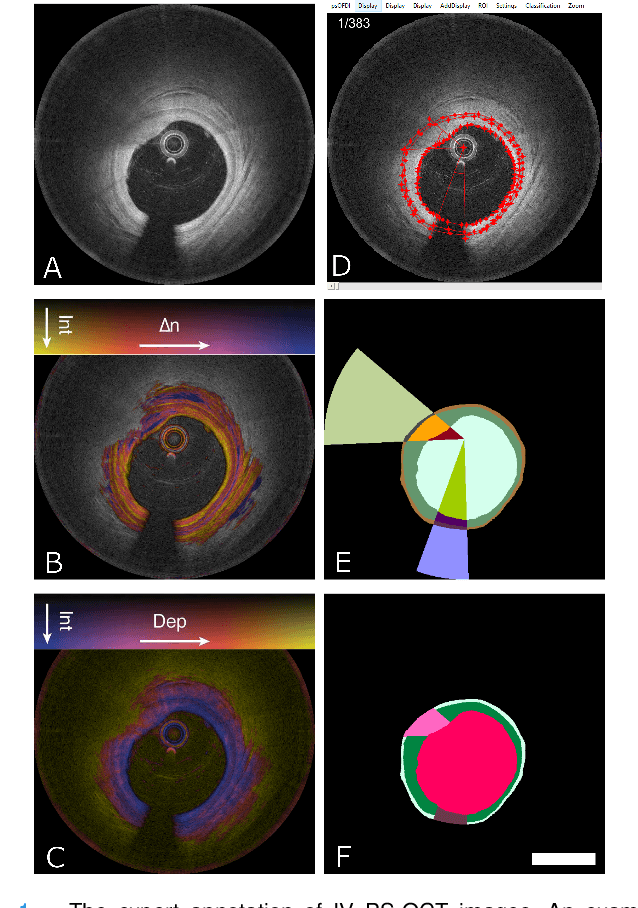
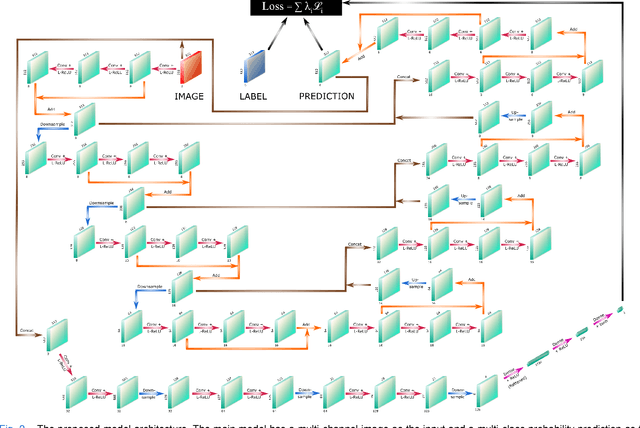
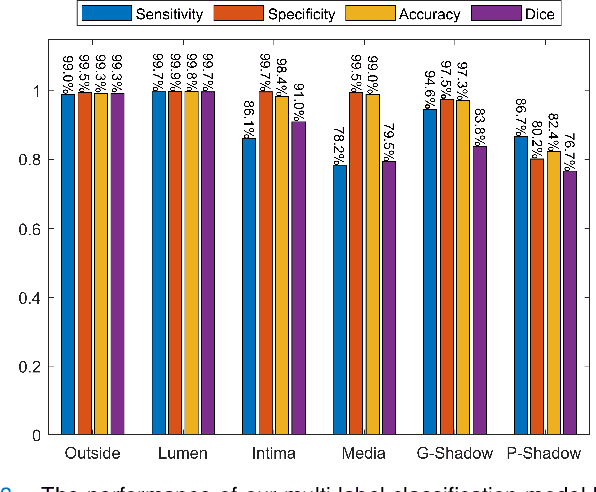
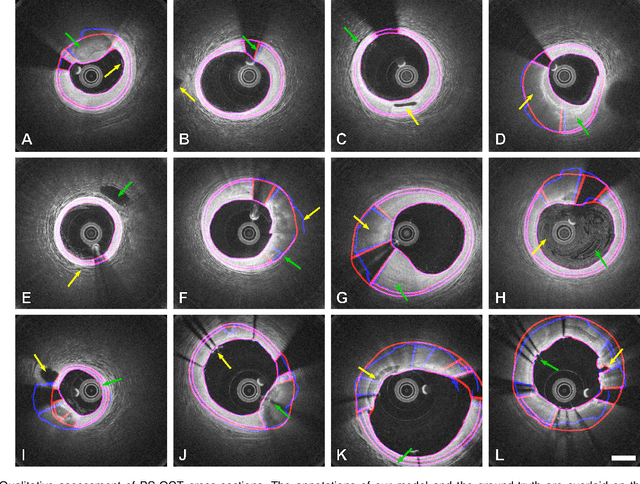
Abstract:Cardiovascular diseases are the leading cause of death and require a spectrum of diagnostic procedures as well as invasive interventions. Medical imaging is a vital part of the healthcare system, facilitating both diagnosis and guidance for intervention. Intravascular ultrasound and optical coherence tomography are widely available for characterizing coronary stenoses and provide critical vessel parameters to optimize percutaneous intervention. Intravascular polarization-sensitive optical coherence tomography (PS-OCT) can simultaneously provide high-resolution cross-sectional images of vascular structures while also revealing preponderant tissue components such as collagen and smooth muscle and thereby enhance plaque characterization. Automated interpretation of these features would facilitate the objective clinical investigation of the natural history and significance of coronary atheromas. Here, we propose a convolutional neural network model and optimize its performance using a new multi-term loss function to classify the lumen, intima, and media layers in addition to the guidewire and plaque artifacts. Our multi-class classification model outperforms the state-of-the-art methods in detecting the anatomical layers based on accuracy, Dice coefficient, and average boundary error. Furthermore, the proposed model segments two classes of major artifacts and detects the anatomical layers within the thickened vessel wall regions, which were excluded from analysis by other studies. The source code and the trained model are publicly available at https://github.com/mhaft/OCTseg .
Dynamic Coronary Roadmapping via Catheter Tip Tracking in X-ray Fluoroscopy with Deep Learning Based Bayesian Filtering
Jan 11, 2020



Abstract:Percutaneous coronary intervention (PCI) is typically performed with image guidance using X-ray angiograms in which coronary arteries are opacified with X-ray opaque contrast agents. Interventional cardiologists typically navigate instruments using non-contrast-enhanced fluoroscopic images, since higher use of contrast agents increases the risk of kidney failure. When using fluoroscopic images, the interventional cardiologist needs to rely on a mental anatomical reconstruction. This paper reports on the development of a novel dynamic coronary roadmapping approach for improving visual feedback and reducing contrast use during PCI. The approach compensates cardiac and respiratory induced vessel motion by ECG alignment and catheter tip tracking in X-ray fluoroscopy, respectively. In particular, for accurate and robust tracking of the catheter tip, we proposed a new deep learning based Bayesian filtering method that integrates the detection outcome of a convolutional neural network and the motion estimation between frames using a particle filtering framework. The proposed roadmapping and tracking approaches were validated on clinical X-ray images, achieving accurate performance on both catheter tip tracking and dynamic coronary roadmapping experiments. In addition, our approach runs in real-time on a computer with a single GPU and has the potential to be integrated into the clinical workflow of PCI procedures, providing cardiologists with visual guidance during interventions without the need of extra use of contrast agent.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge