Mohammad Haft-Javaherian
Segmentation of Anatomical Layers and Artifacts in Intravascular Polarization Sensitive Optical Coherence Tomography Using Attending Physician and Boundary Cardinality Lost Terms
May 11, 2021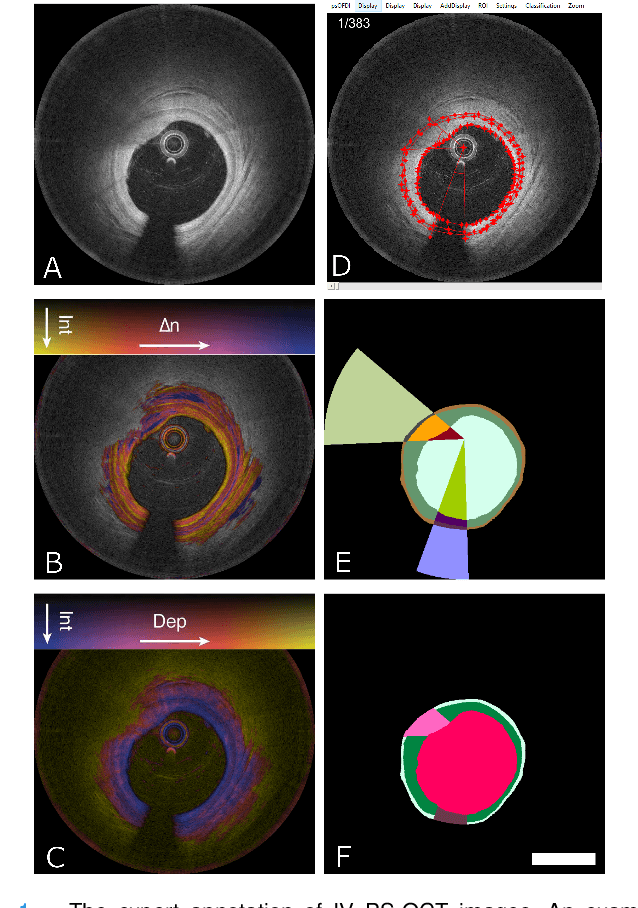
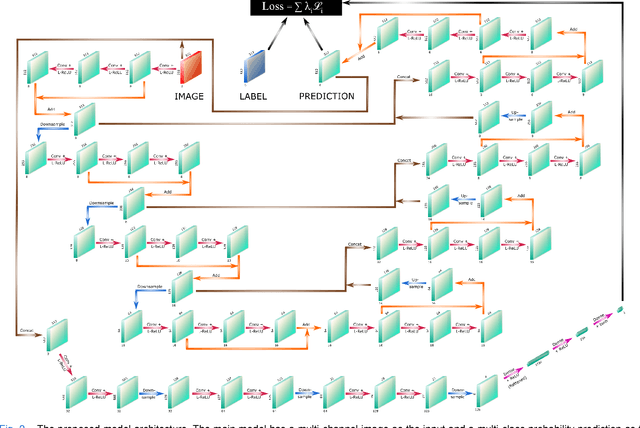
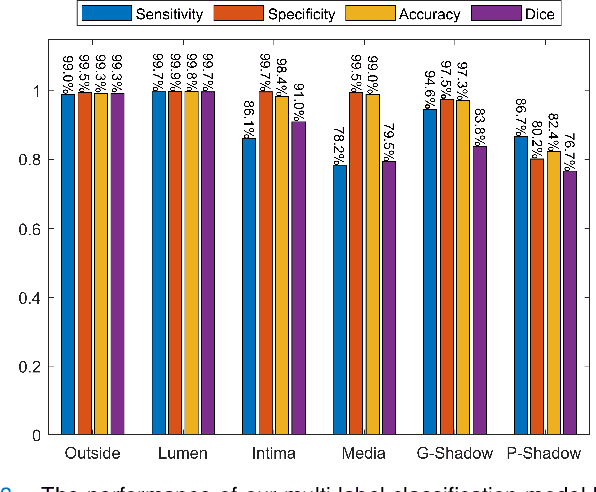
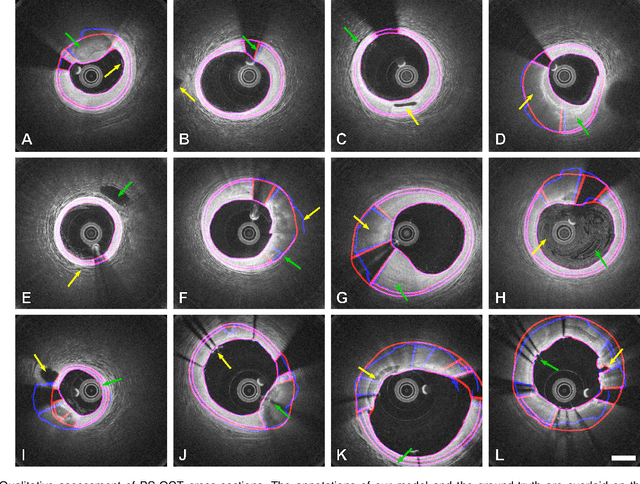
Abstract:Cardiovascular diseases are the leading cause of death and require a spectrum of diagnostic procedures as well as invasive interventions. Medical imaging is a vital part of the healthcare system, facilitating both diagnosis and guidance for intervention. Intravascular ultrasound and optical coherence tomography are widely available for characterizing coronary stenoses and provide critical vessel parameters to optimize percutaneous intervention. Intravascular polarization-sensitive optical coherence tomography (PS-OCT) can simultaneously provide high-resolution cross-sectional images of vascular structures while also revealing preponderant tissue components such as collagen and smooth muscle and thereby enhance plaque characterization. Automated interpretation of these features would facilitate the objective clinical investigation of the natural history and significance of coronary atheromas. Here, we propose a convolutional neural network model and optimize its performance using a new multi-term loss function to classify the lumen, intima, and media layers in addition to the guidewire and plaque artifacts. Our multi-class classification model outperforms the state-of-the-art methods in detecting the anatomical layers based on accuracy, Dice coefficient, and average boundary error. Furthermore, the proposed model segments two classes of major artifacts and detects the anatomical layers within the thickened vessel wall regions, which were excluded from analysis by other studies. The source code and the trained model are publicly available at https://github.com/mhaft/OCTseg .
Deep convolutional neural networks for segmenting 3D in vivo multiphoton images of vasculature in Alzheimer disease mouse models
Oct 17, 2018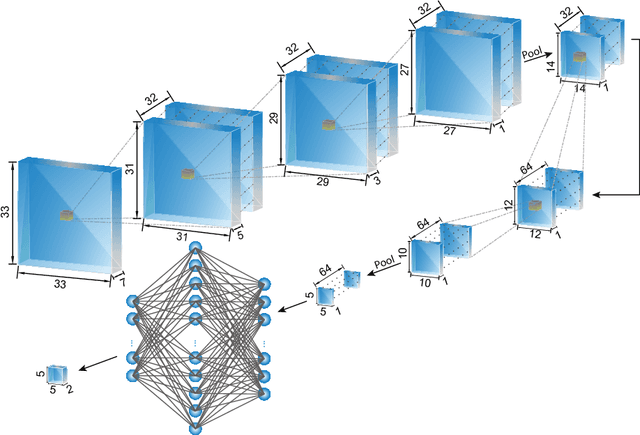
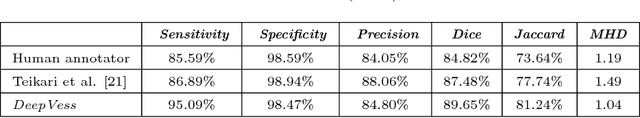


Abstract:The health and function of tissue rely on its vasculature network to provide reliable blood perfusion. Volumetric imaging approaches, such as multiphoton microscopy, are able to generate detailed 3D images of blood vessels that could contribute to our understanding of the role of vascular structure in normal physiology and in disease mechanisms. The segmentation of vessels, a core image analysis problem, is a bottleneck that has prevented the systematic comparison of 3D vascular architecture across experimental populations. We explored the use of convolutional neural networks to segment 3D vessels within volumetric in vivo images acquired by multiphoton microscopy. We evaluated different network architectures and machine learning techniques in the context of this segmentation problem. We show that our optimized convolutional neural network architecture, which we call DeepVess, yielded a segmentation accuracy that was better than both the current state-of-the-art and a trained human annotator, while also being orders of magnitude faster. To explore the effects of aging and Alzheimer's disease on capillaries, we applied DeepVess to 3D images of cortical blood vessels in young and old mouse models of Alzheimer's disease and wild type littermates. We found little difference in the distribution of capillary diameter or tortuosity between these groups, but did note a decrease in the number of longer capillary segments ($>75\mu m$) in aged animals as compared to young, in both wild type and Alzheimer's disease mouse models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge