Brett E. Bouma
Probabilistic volumetric speckle suppression in OCT using deep learning
Dec 07, 2023Abstract:We present a deep learning framework for volumetric speckle reduction in optical coherence tomography (OCT) based on a conditional generative adversarial network (cGAN) that leverages the volumetric nature of OCT data. In order to utilize the volumetric nature of OCT data, our network takes partial OCT volumes as input, resulting in artifact-free despeckled volumes that exhibit excellent speckle reduction and resolution preservation in all three dimensions. Furthermore, we address the ongoing challenge of generating ground truth data for supervised speckle suppression deep learning frameworks by using volumetric non-local means despeckling-TNode to generate training data. We show that, while TNode processing is computationally demanding, it serves as a convenient, accessible gold-standard source for training data; our cGAN replicates efficient suppression of speckle while preserving tissue structures with dimensions approaching the system resolution of non-local means despeckling while being two orders of magnitude faster than TNode. We demonstrate fast, effective, and high-quality despeckling of the proposed network in different tissue types acquired with three different OCT systems compared to existing deep learning methods. The open-source nature of our work facilitates re-training and deployment in any OCT system with an all-software implementation, working around the challenge of generating high-quality, speckle-free training data.
Single-Input Polarization-Sensitive Optical Coherence Tomography Through a Catheter
May 16, 2023Abstract:Intravascular polarimetry with catheter-based polarization-sensitive optical coherence tomography (PS-OCT) complements the high-resolution structural tomograms of OCT with morphological contrast available through polarimetry. Its clinical translation has been complicated by the need for modification of conventional OCT hardware to enable polarimetric measurements. Here, we present a signal processing method to reconstruct polarization properties of tissue from measurements with a single input polarization state, bypassing the need for modulation or multiplexing of input states. Our method relies on a polarization symmetry intrinsic to round-trip measurements and uses the residual spectral variation of the polarization states incident on the tissue to avoid measurement ambiguities. We demonstrate depth-resolved birefringence and optic axis orientation maps reconstructed from in-vivo data of human coronary arteries. We validate our method through comparison with conventional dual-input state measurements and find a mean cumulative retardance error of 13.2deg without observable bias. The 95% limit of agreement between depth-resolved birefringence is 2.80 x 10^(-4), which is less than the agreement between two repeat pullbacks of conventional PS-OCT (3.14 x 10^(-4)), indicating that the two methods can be used interchangeably. The hardware simplification arising from using a single input state may be decisive in realizing the potential of polarimetric measurements for assessing coronary atherosclerosis in clinical practice.
Segmentation of Anatomical Layers and Artifacts in Intravascular Polarization Sensitive Optical Coherence Tomography Using Attending Physician and Boundary Cardinality Lost Terms
May 11, 2021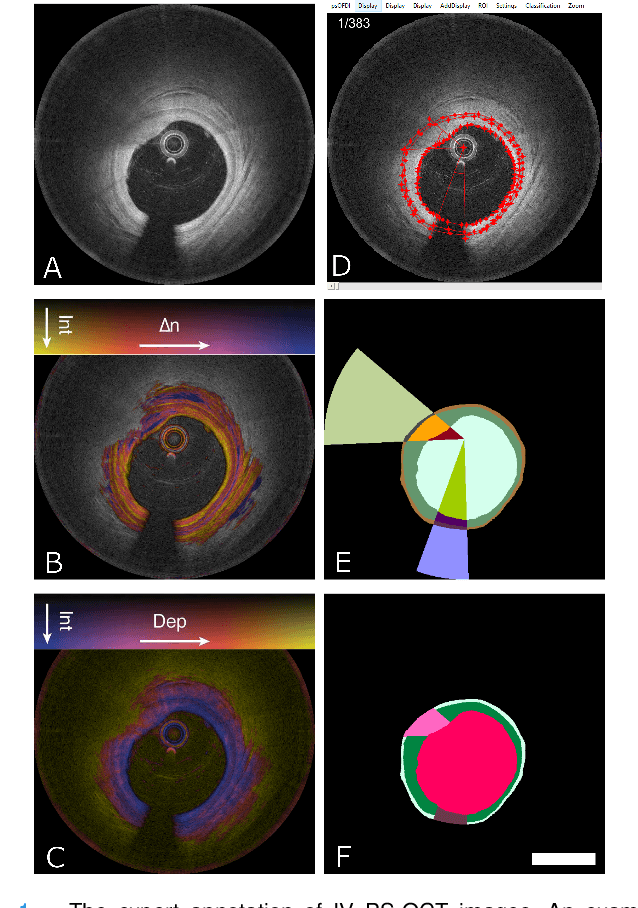
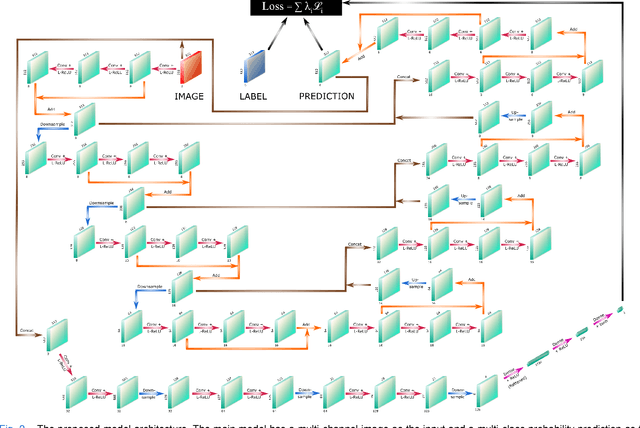
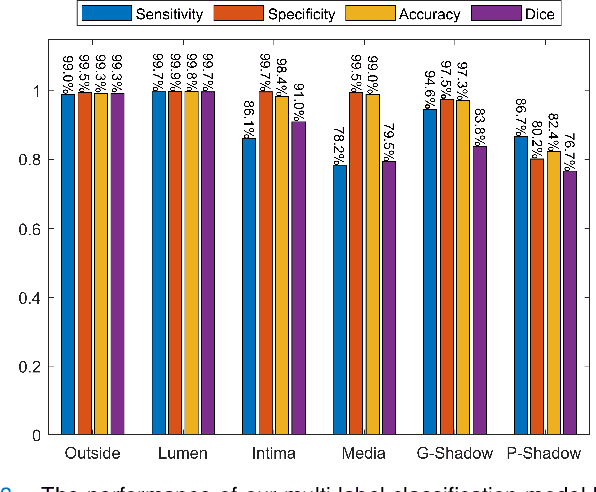
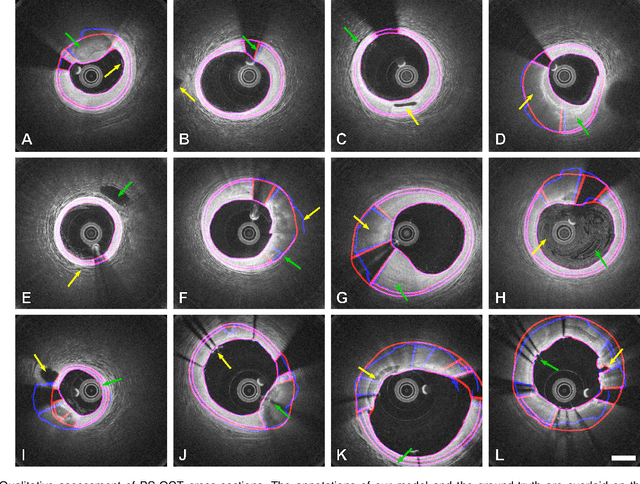
Abstract:Cardiovascular diseases are the leading cause of death and require a spectrum of diagnostic procedures as well as invasive interventions. Medical imaging is a vital part of the healthcare system, facilitating both diagnosis and guidance for intervention. Intravascular ultrasound and optical coherence tomography are widely available for characterizing coronary stenoses and provide critical vessel parameters to optimize percutaneous intervention. Intravascular polarization-sensitive optical coherence tomography (PS-OCT) can simultaneously provide high-resolution cross-sectional images of vascular structures while also revealing preponderant tissue components such as collagen and smooth muscle and thereby enhance plaque characterization. Automated interpretation of these features would facilitate the objective clinical investigation of the natural history and significance of coronary atheromas. Here, we propose a convolutional neural network model and optimize its performance using a new multi-term loss function to classify the lumen, intima, and media layers in addition to the guidewire and plaque artifacts. Our multi-class classification model outperforms the state-of-the-art methods in detecting the anatomical layers based on accuracy, Dice coefficient, and average boundary error. Furthermore, the proposed model segments two classes of major artifacts and detects the anatomical layers within the thickened vessel wall regions, which were excluded from analysis by other studies. The source code and the trained model are publicly available at https://github.com/mhaft/OCTseg .
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge