Joelle Hallak
Contrastive learning-based pretraining improves representation and transferability of diabetic retinopathy classification models
Aug 24, 2022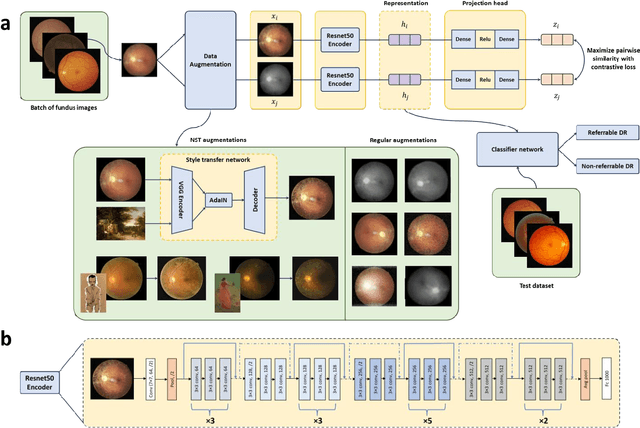
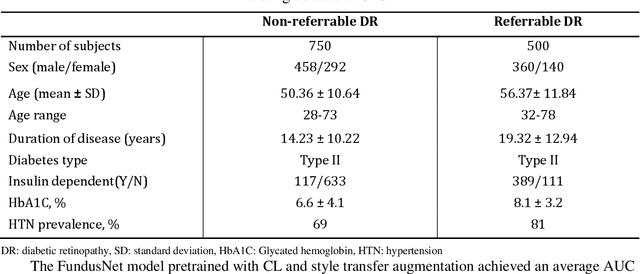
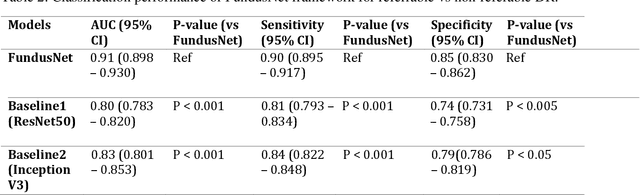
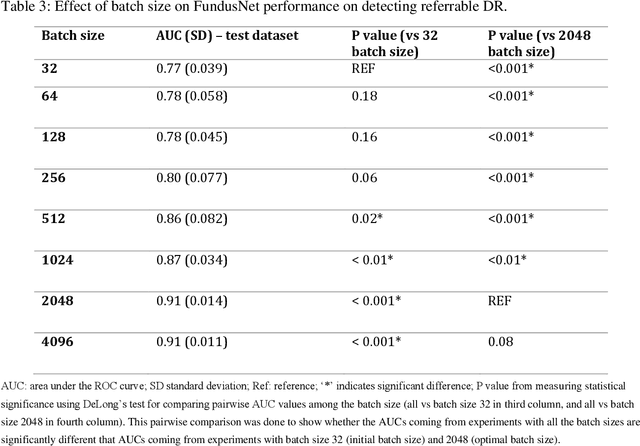
Abstract:Self supervised contrastive learning based pretraining allows development of robust and generalized deep learning models with small, labeled datasets, reducing the burden of label generation. This paper aims to evaluate the effect of CL based pretraining on the performance of referrable vs non referrable diabetic retinopathy (DR) classification. We have developed a CL based framework with neural style transfer (NST) augmentation to produce models with better representations and initializations for the detection of DR in color fundus images. We compare our CL pretrained model performance with two state of the art baseline models pretrained with Imagenet weights. We further investigate the model performance with reduced labeled training data (down to 10 percent) to test the robustness of the model when trained with small, labeled datasets. The model is trained and validated on the EyePACS dataset and tested independently on clinical data from the University of Illinois, Chicago (UIC). Compared to baseline models, our CL pretrained FundusNet model had higher AUC (CI) values (0.91 (0.898 to 0.930) vs 0.80 (0.783 to 0.820) and 0.83 (0.801 to 0.853) on UIC data). At 10 percent labeled training data, the FundusNet AUC was 0.81 (0.78 to 0.84) vs 0.58 (0.56 to 0.64) and 0.63 (0.60 to 0.66) in baseline models, when tested on the UIC dataset. CL based pretraining with NST significantly improves DL classification performance, helps the model generalize well (transferable from EyePACS to UIC data), and allows training with small, annotated datasets, therefore reducing ground truth annotation burden of the clinicians.
A Deep-learning Approach for Prognosis of Age-Related Macular Degeneration Disease using SD-OCT Imaging Biomarkers
Feb 27, 2019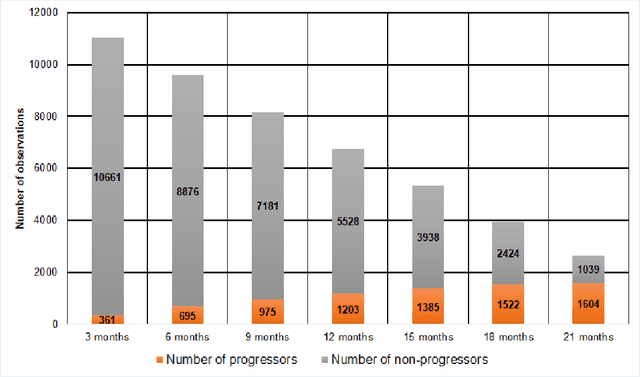
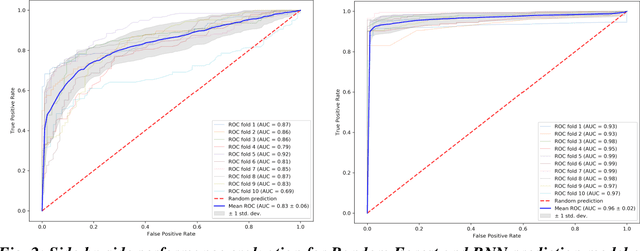
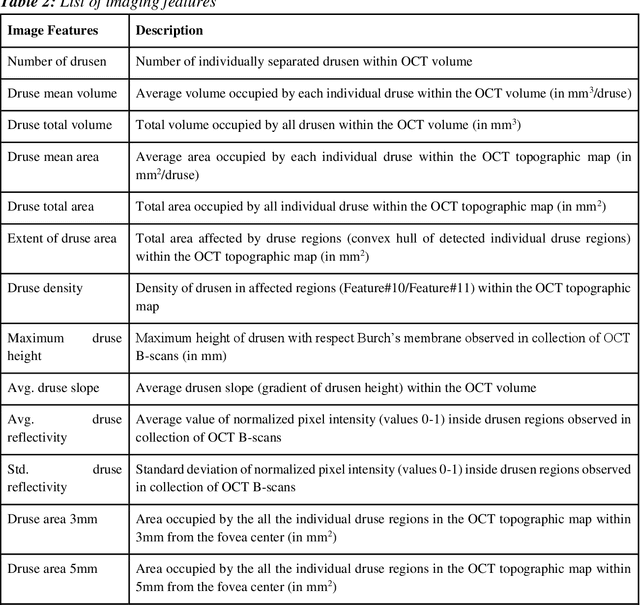
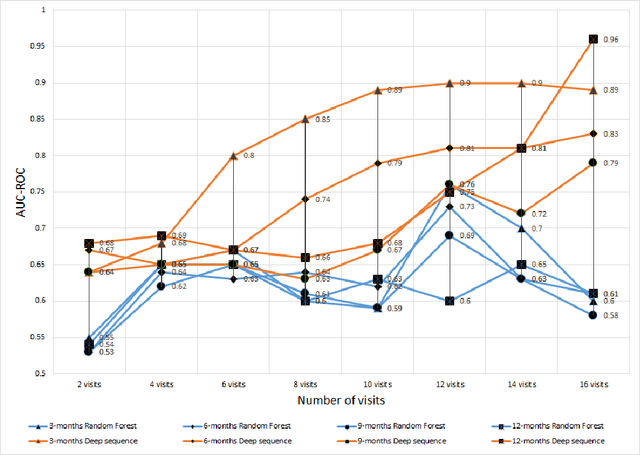
Abstract:We propose a hybrid sequential deep learning model to predict the risk of AMD progression in non-exudative AMD eyes at multiple timepoints, starting from short-term progression (3-months) up to long-term progression (21-months). Proposed model combines radiomics and deep learning to handle challenges related to imperfect ratio of OCT scan dimension and training cohort size. We considered a retrospective clinical trial dataset that includes 671 fellow eyes with 13,954 dry AMD observations for training and validating the machine learning models on a 10-fold cross validation setting. The proposed RNN model achieved high accuracy (0.96 AUCROC) for the prediction of both short term and long-term AMD progression, and outperformed the traditional random forest model trained. High accuracy achieved by the RNN establishes the ability to identify AMD patients at risk of progressing to advanced AMD at an early stage which could have a high clinical impact as it allows for optimal clinical follow-up, with more frequent screening and potential earlier treatment for those patients at high risk.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge