Joel Jaskari
Temporal social network modeling of mobile connectivity data with graph neural networks
Sep 03, 2025Abstract:Graph neural networks (GNNs) have emerged as a state-of-the-art data-driven tool for modeling connectivity data of graph-structured complex networks and integrating information of their nodes and edges in space and time. However, as of yet, the analysis of social networks using the time series of people's mobile connectivity data has not been extensively investigated. In the present study, we investigate four snapshot - based temporal GNNs in predicting the phone call and SMS activity between users of a mobile communication network. In addition, we develop a simple non - GNN baseline model using recently proposed EdgeBank method. Our analysis shows that the ROLAND temporal GNN outperforms the baseline model in most cases, whereas the other three GNNs perform on average worse than the baseline. The results show that GNN based approaches hold promise in the analysis of temporal social networks through mobile connectivity data. However, due to the relatively small performance margin between ROLAND and the baseline model, further research is required on specialized GNN architectures for temporal social network analysis.
Interactive 3D Segmentation for Primary Gross Tumor Volume in Oropharyngeal Cancer
Sep 10, 2024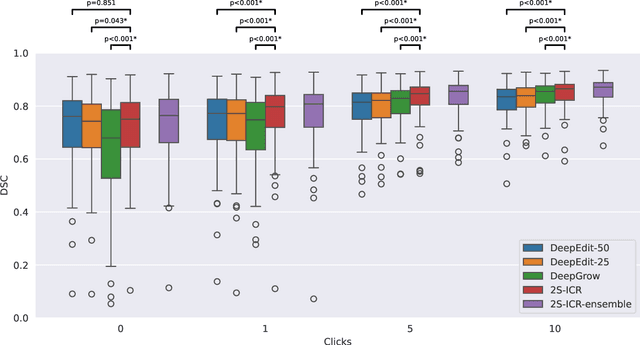
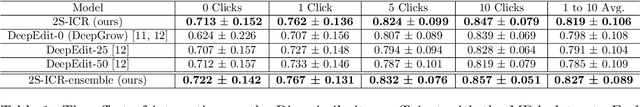

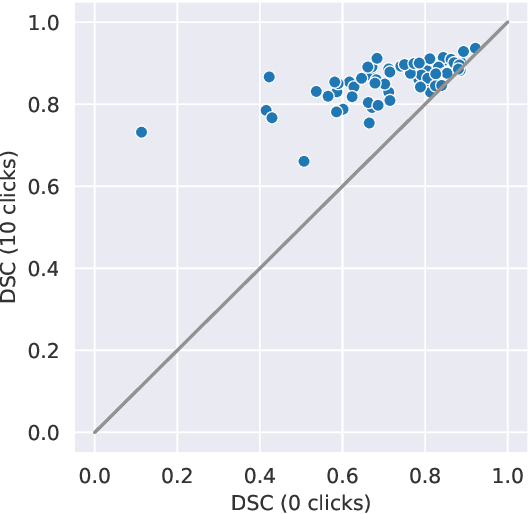
Abstract:The main treatment modality for oropharyngeal cancer (OPC) is radiotherapy, where accurate segmentation of the primary gross tumor volume (GTVp) is essential. However, accurate GTVp segmentation is challenging due to significant interobserver variability and the time-consuming nature of manual annotation, while fully automated methods can occasionally fail. An interactive deep learning (DL) model offers the advantage of automatic high-performance segmentation with the flexibility for user correction when necessary. In this study, we examine interactive DL for GTVp segmentation in OPC. We implement state-of-the-art algorithms and propose a novel two-stage Interactive Click Refinement (2S-ICR) framework. Using the 2021 HEad and neCK TumOR (HECKTOR) dataset for development and an external dataset from The University of Texas MD Anderson Cancer Center for evaluation, the 2S-ICR framework achieves a Dice similarity coefficient of 0.713 $\pm$ 0.152 without user interaction and 0.824 $\pm$ 0.099 after five interactions, outperforming existing methods in both cases.
Comparison of Deep Learning Segmentation and Multigrader-annotated Mandibular Canals of Multicenter CBCT scans
May 27, 2022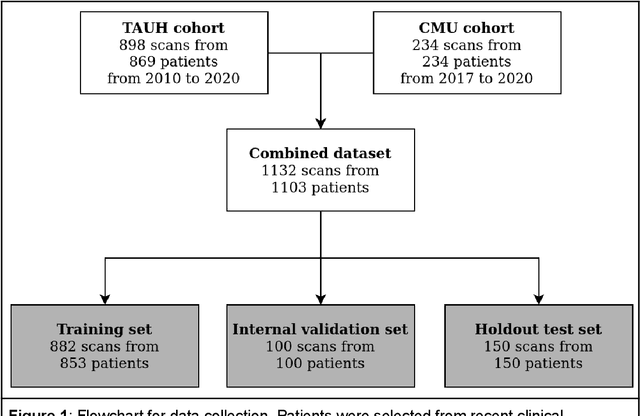
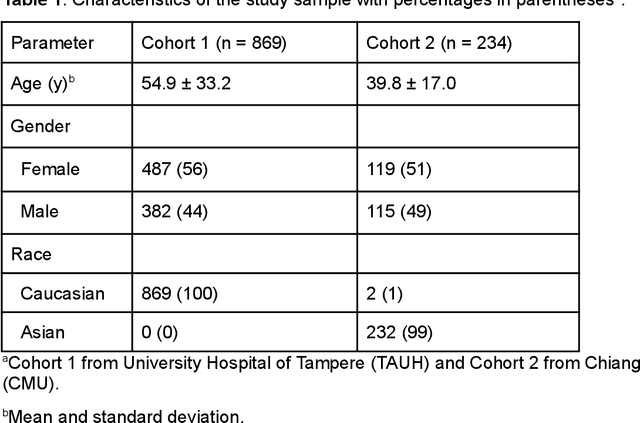
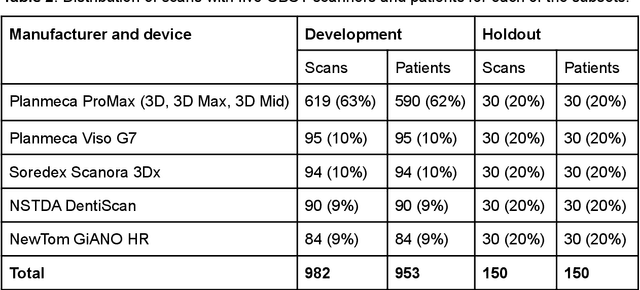
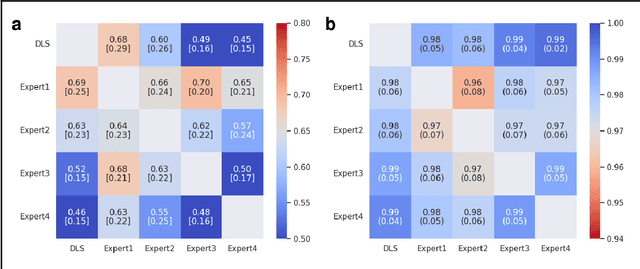
Abstract:Deep learning approach has been demonstrated to automatically segment the bilateral mandibular canals from CBCT scans, yet systematic studies of its clinical and technical validation are scarce. To validate the mandibular canal localization accuracy of a deep learning system (DLS) we trained it with 982 CBCT scans and evaluated using 150 scans of five scanners from clinical workflow patients of European and Southeast Asian Institutes, annotated by four radiologists. The interobserver variability was compared to the variability between the DLS and the radiologists. In addition, the generalization of DLS to CBCT scans from scanners not used in the training data was examined to evaluate the out-of-distribution generalization capability. The DLS had lower variability to the radiologists than the interobserver variability between them and it was able to generalize to three new devices. For the radiologists' consensus segmentation, used as gold standard, the DLS had a symmetric mean curve distance of 0.39 mm compared to those of the individual radiologists with 0.62 mm, 0.55 mm, 0.47 mm, and 0.42 mm. The DLS showed comparable or slightly better performance in the segmentation of the mandibular canal with the radiologists and generalization capability to new scanners.
Uncertainty-aware deep learning methods for robust diabetic retinopathy classification
Feb 02, 2022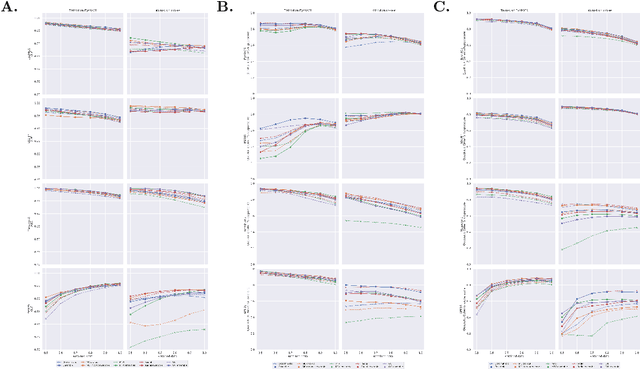
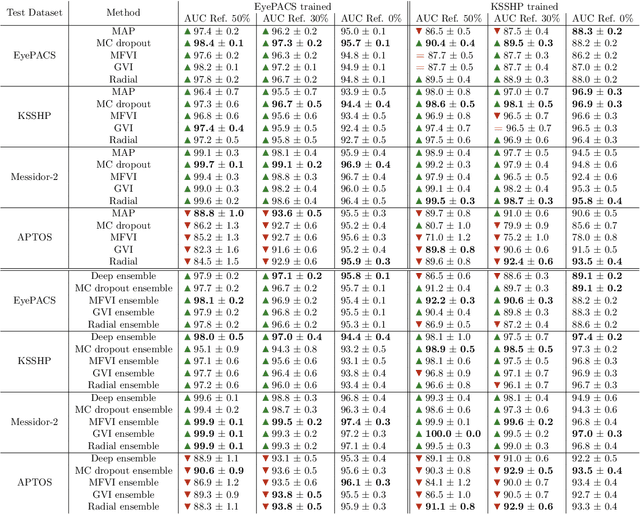
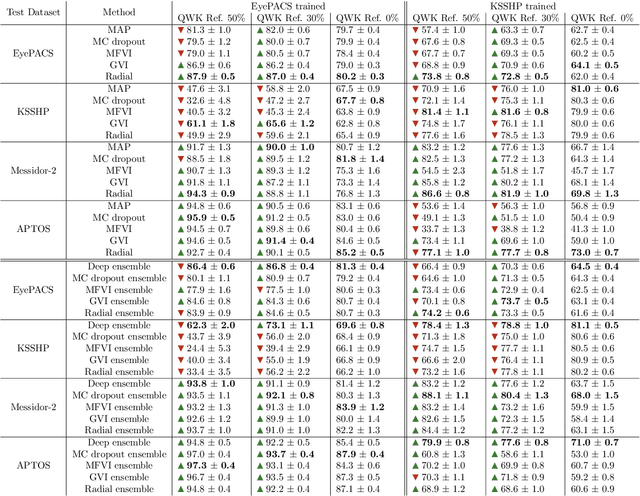
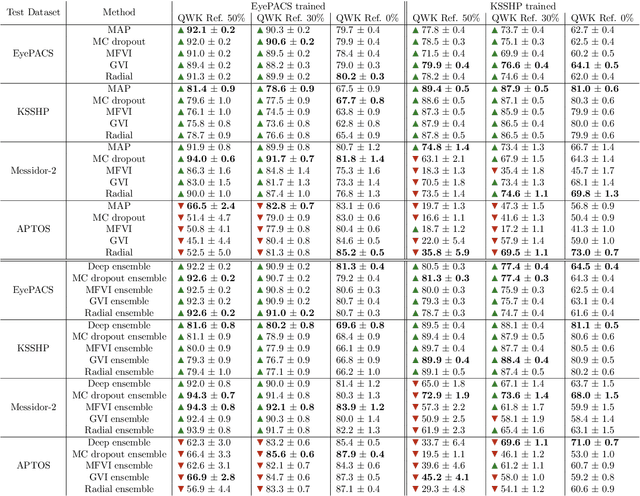
Abstract:Automatic classification of diabetic retinopathy from retinal images has been widely studied using deep neural networks with impressive results. However, there is a clinical need for estimation of the uncertainty in the classifications, a shortcoming of modern neural networks. Recently, approximate Bayesian deep learning methods have been proposed for the task but the studies have only considered the binary referable/non-referable diabetic retinopathy classification applied to benchmark datasets. We present novel results by systematically investigating a clinical dataset and a clinically relevant 5-class classification scheme, in addition to benchmark datasets and the binary classification scheme. Moreover, we derive a connection between uncertainty measures and classifier risk, from which we develop a new uncertainty measure. We observe that the previously proposed entropy-based uncertainty measure generalizes to the clinical dataset on the binary classification scheme but not on the 5-class scheme, whereas our new uncertainty measure generalizes to the latter case.
Deep Learning Fundus Image Analysis for Diabetic Retinopathy and Macular Edema Grading
Apr 16, 2019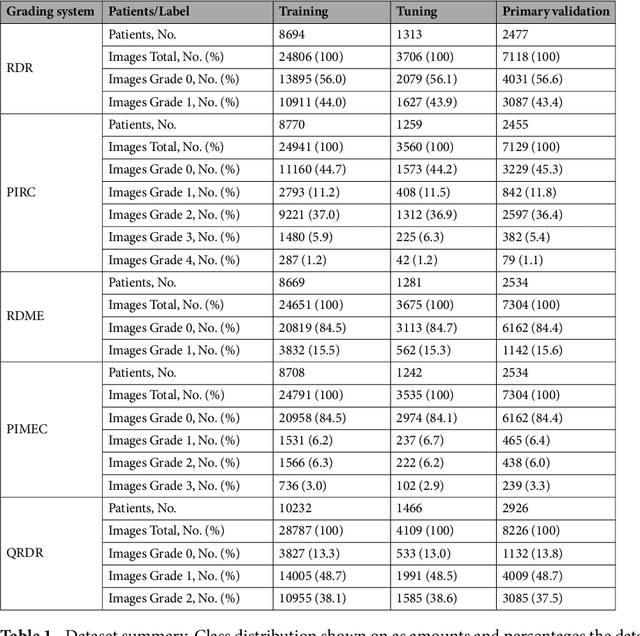
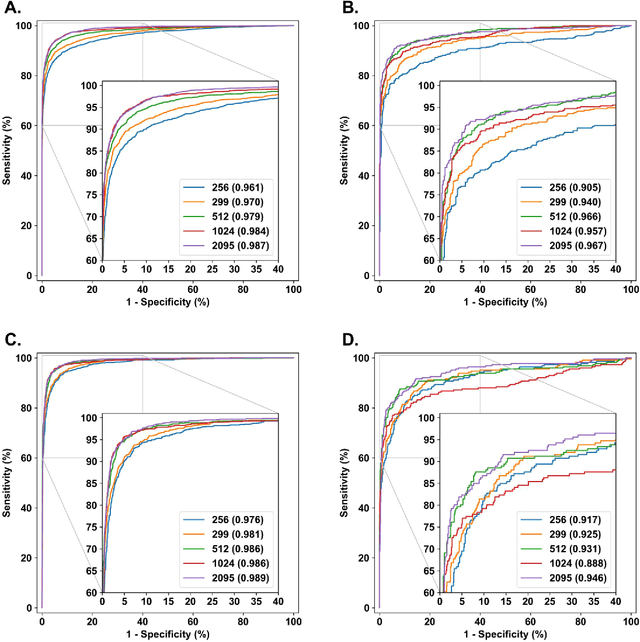
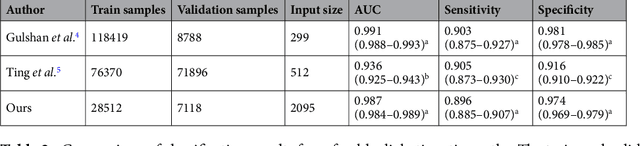
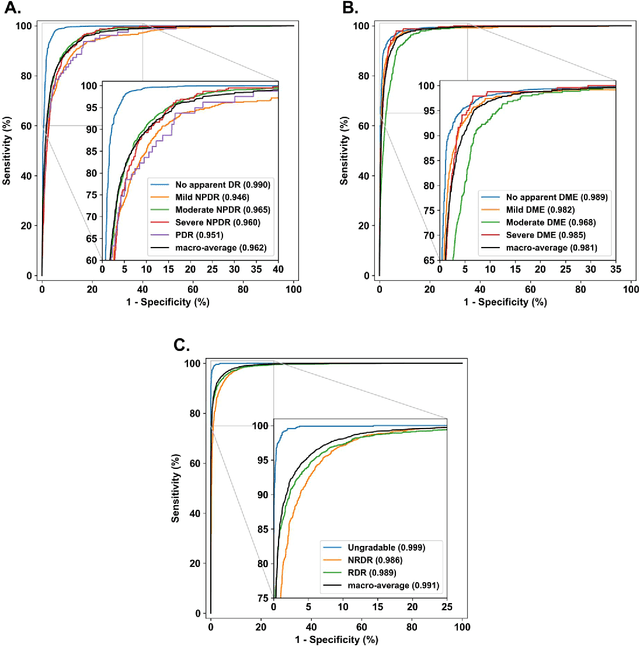
Abstract:Diabetes is a globally prevalent disease that can cause visible microvascular complications such as diabetic retinopathy and macular edema in the human eye retina, the images of which are today used for manual disease screening. This labor-intensive task could greatly benefit from automatic detection using deep learning technique. Here we present a deep learning system that identifies referable diabetic retinopathy comparably or better than presented in the previous studies, although we use only a small fraction of images (<1/4) in training but are aided with higher image resolutions. We also provide novel results for five different screening and clinical grading systems for diabetic retinopathy and macular edema classification, including results for accurately classifying images according to clinical five-grade diabetic retinopathy and four-grade diabetic macular edema scales. These results suggest, that a deep learning system could increase the cost-effectiveness of screening while attaining higher than recommended performance, and that the system could be applied in clinical examinations requiring finer grading.
A Novel Variational Autoencoder with Applications to Generative Modelling, Classification, and Ordinal Regression
Dec 19, 2018



Abstract:We develop a novel probabilistic generative model based on the variational autoencoder approach. Notable aspects of our architecture are: a novel way of specifying the latent variables prior, and the introduction of an ordinality enforcing unit. We describe how to do supervised, unsupervised and semi-supervised learning, and nominal and ordinal classification, with the model. We analyze generative properties of the approach, and the classification effectiveness under nominal and ordinal classification, using two benchmark datasets. Our results show that our model can achieve comparable results with relevant baselines in both of the classification tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge