Jingna Feng
Early GVHD Prediction in Liver Transplantation via Multi-Modal Deep Learning on Imbalanced EHR Data
Nov 06, 2025Abstract:Graft-versus-host disease (GVHD) is a rare but often fatal complication in liver transplantation, with a very high mortality rate. By harnessing multi-modal deep learning methods to integrate heterogeneous and imbalanced electronic health records (EHR), we aim to advance early prediction of GVHD, paving the way for timely intervention and improved patient outcomes. In this study, we analyzed pre-transplant electronic health records (EHR) spanning the period before surgery for 2,100 liver transplantation patients, including 42 cases of graft-versus-host disease (GVHD), from a cohort treated at Mayo Clinic between 1992 and 2025. The dataset comprised four major modalities: patient demographics, laboratory tests, diagnoses, and medications. We developed a multi-modal deep learning framework that dynamically fuses these modalities, handles irregular records with missing values, and addresses extreme class imbalance through AUC-based optimization. The developed framework outperforms all single-modal and multi-modal machine learning baselines, achieving an AUC of 0.836, an AUPRC of 0.157, a recall of 0.768, and a specificity of 0.803. It also demonstrates the effectiveness of our approach in capturing complementary information from different modalities, leading to improved performance. Our multi-modal deep learning framework substantially improves existing approaches for early GVHD prediction. By effectively addressing the challenges of heterogeneity and extreme class imbalance in real-world EHR, it achieves accurate early prediction. Our proposed multi-modal deep learning method demonstrates promising results for early prediction of a GVHD in liver transplantation, despite the challenge of extremely imbalanced EHR data.
Explainable Graph Neural Network for Alzheimer's Disease And Related Dementias Risk Prediction
Sep 18, 2023Abstract:Alzheimer's disease and related dementias (ADRD) ranks as the sixth leading cause of death in the US, underlining the importance of accurate ADRD risk prediction. While recent advancement in ADRD risk prediction have primarily relied on imaging analysis, yet not all patients undergo medical imaging before an ADRD diagnosis. Merging machine learning with claims data can reveal additional risk factors and uncover interconnections among diverse medical codes. Our goal is to utilize Graph Neural Networks (GNNs) with claims data for ADRD risk prediction. Addressing the lack of human-interpretable reasons behind these predictions, we introduce an innovative method to evaluate relationship importance and its influence on ADRD risk prediction, ensuring comprehensive interpretation. We employed Variationally Regularized Encoder-decoder Graph Neural Network (VGNN) for estimating ADRD likelihood. We created three scenarios to assess the model's efficiency, using Random Forest and Light Gradient Boost Machine as baselines. We further used our relation importance method to clarify the key relationships for ADRD risk prediction. VGNN surpassed other baseline models by 10% in the area under the receiver operating characteristic. The integration of the GNN model and relation importance interpretation could potentially play an essential role in providing valuable insight into factors that may contribute to or delay ADRD progression. Employing a GNN approach with claims data enhances ADRD risk prediction and provides insights into the impact of interconnected medical code relationships. This methodology not only enables ADRD risk modeling but also shows potential for other image analysis predictions using claims data.
Mining On Alzheimer's Diseases Related Knowledge Graph to Identity Potential AD-related Semantic Triples for Drug Repurposing
Feb 17, 2022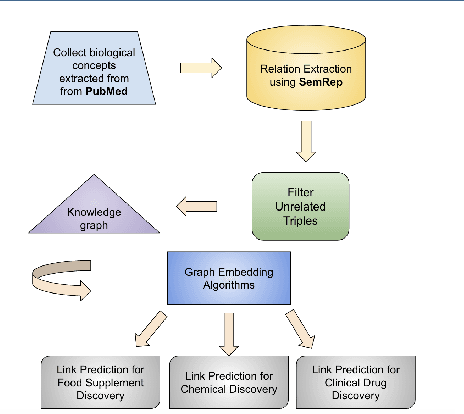

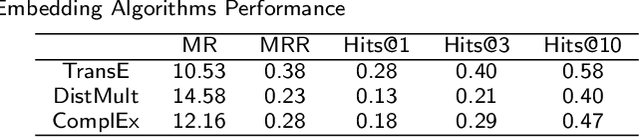
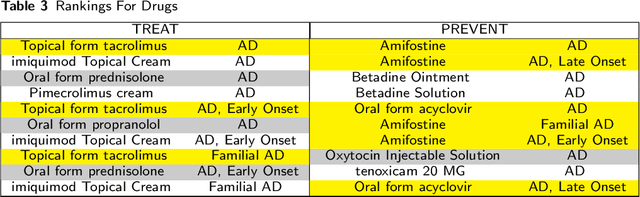
Abstract:To date, there are no effective treatments for most neurodegenerative diseases. Knowledge graphs can provide comprehensive and semantic representation for heterogeneous data, and have been successfully leveraged in many biomedical applications including drug repurposing. Our objective is to construct a knowledge graph from literature to study relations between Alzheimer's disease (AD) and chemicals, drugs and dietary supplements in order to identify opportunities to prevent or delay neurodegenerative progression. We collected biomedical annotations and extracted their relations using SemRep via SemMedDB. We used both a BERT-based classifier and rule-based methods during data preprocessing to exclude noise while preserving most AD-related semantic triples. The 1,672,110 filtered triples were used to train with knowledge graph completion algorithms (i.e., TransE, DistMult, and ComplEx) to predict candidates that might be helpful for AD treatment or prevention. Among three knowledge graph completion models, TransE outperformed the other two (MR = 13.45, Hits@1 = 0.306). We leveraged the time-slicing technique to further evaluate the prediction results. We found supporting evidence for most highly ranked candidates predicted by our model which indicates that our approach can inform reliable new knowledge. This paper shows that our graph mining model can predict reliable new relationships between AD and other entities (i.e., dietary supplements, chemicals, and drugs). The knowledge graph constructed can facilitate data-driven knowledge discoveries and the generation of novel hypotheses.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge