Jianqi Sun
U2AD: Uncertainty-based Unsupervised Anomaly Detection Framework for Detecting T2 Hyperintensity in MRI Spinal Cord
Mar 17, 2025Abstract:T2 hyperintensities in spinal cord MR images are crucial biomarkers for conditions such as degenerative cervical myelopathy. However, current clinical diagnoses primarily rely on manual evaluation. Deep learning methods have shown promise in lesion detection, but most supervised approaches are heavily dependent on large, annotated datasets. Unsupervised anomaly detection (UAD) offers a compelling alternative by eliminating the need for abnormal data annotations. However, existing UAD methods rely on curated normal datasets and their performance frequently deteriorates when applied to clinical datasets due to domain shifts. We propose an Uncertainty-based Unsupervised Anomaly Detection framework, termed U2AD, to address these limitations. Unlike traditional methods, U2AD is designed to be trained and tested within the same clinical dataset, following a "mask-and-reconstruction" paradigm built on a Vision Transformer-based architecture. We introduce an uncertainty-guided masking strategy to resolve task conflicts between normal reconstruction and anomaly detection to achieve an optimal balance. Specifically, we employ a Monte-Carlo sampling technique to estimate reconstruction uncertainty mappings during training. By iteratively optimizing reconstruction training under the guidance of both epistemic and aleatoric uncertainty, U2AD reduces overall reconstruction variance while emphasizing regions. Experimental results demonstrate that U2AD outperforms existing supervised and unsupervised methods in patient-level identification and segment-level localization tasks. This framework establishes a new benchmark for incorporating uncertainty guidance into UAD, highlighting its clinical utility in addressing domain shifts and task conflicts in medical image anomaly detection. Our code is available: https://github.com/zhibaishouheilab/U2AD
HealthiVert-GAN: A Novel Framework of Pseudo-Healthy Vertebral Image Synthesis for Interpretable Compression Fracture Grading
Mar 08, 2025Abstract:Osteoporotic vertebral compression fractures (VCFs) are prevalent in the elderly population, typically assessed on computed tomography (CT) scans by evaluating vertebral height loss. This assessment helps determine the fracture's impact on spinal stability and the need for surgical intervention. However, clinical data indicate that many VCFs exhibit irregular compression, complicating accurate diagnosis. While deep learning methods have shown promise in aiding VCFs screening, they often lack interpretability and sufficient sensitivity, limiting their clinical applicability. To address these challenges, we introduce a novel vertebra synthesis-height loss quantification-VCFs grading framework. Our proposed model, HealthiVert-GAN, utilizes a coarse-to-fine synthesis network designed to generate pseudo-healthy vertebral images that simulate the pre-fracture state of fractured vertebrae. This model integrates three auxiliary modules that leverage the morphology and height information of adjacent healthy vertebrae to ensure anatomical consistency. Additionally, we introduce the Relative Height Loss of Vertebrae (RHLV) as a quantification metric, which divides each vertebra into three sections to measure height loss between pre-fracture and post-fracture states, followed by fracture severity classification using a Support Vector Machine (SVM). Our approach achieves state-of-the-art classification performance on both the Verse2019 dataset and our private dataset, and it provides cross-sectional distribution maps of vertebral height loss. This practical tool enhances diagnostic sensitivity in clinical settings and assisting in surgical decision-making. Our code is available: https://github.com/zhibaishouheilab/HealthiVert-GAN.
Pathology-Guided AI System for Accurate Segmentation and Diagnosis of Cervical Spondylosis
Mar 08, 2025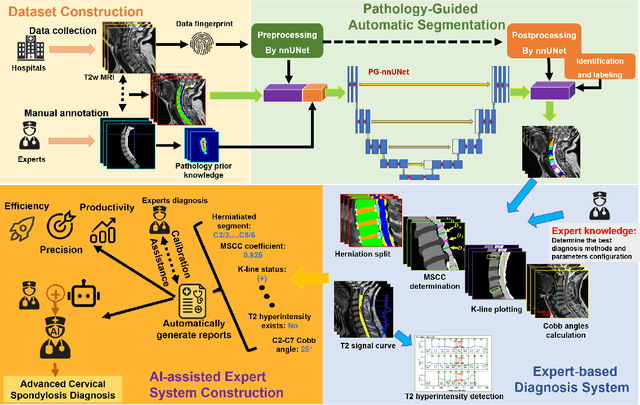
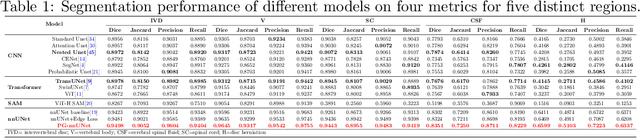
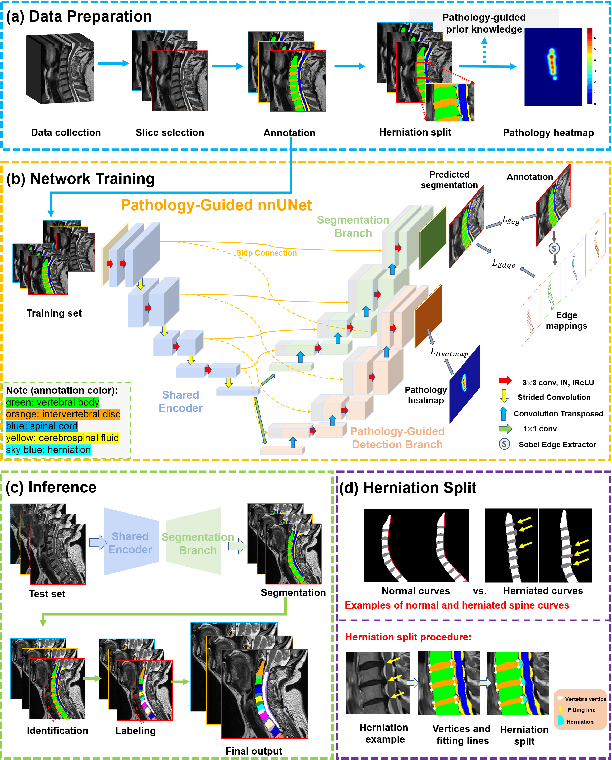
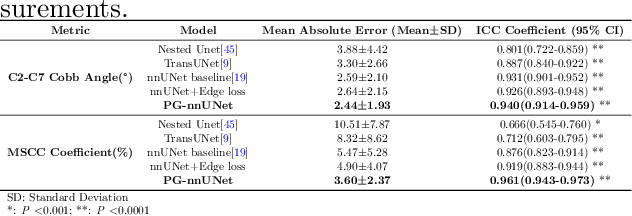
Abstract:Cervical spondylosis, a complex and prevalent condition, demands precise and efficient diagnostic techniques for accurate assessment. While MRI offers detailed visualization of cervical spine anatomy, manual interpretation remains labor-intensive and prone to error. To address this, we developed an innovative AI-assisted Expert-based Diagnosis System that automates both segmentation and diagnosis of cervical spondylosis using MRI. Leveraging a dataset of 960 cervical MRI images from patients with cervical disc herniation, our system features a pathology-guided segmentation model capable of accurately segmenting key cervical anatomical structures. The segmentation is followed by an expert-based diagnostic framework that automates the calculation of critical clinical indicators. Our segmentation model achieved an impressive average Dice coefficient exceeding 0.90 across four cervical spinal anatomies and demonstrated enhanced accuracy in herniation areas. Diagnostic evaluation further showcased the system precision, with a mean absolute error (MAE) of 2.44 degree for the C2-C7 Cobb angle and 3.60 precentage for the Maximum Spinal Cord Compression (MSCC) coefficient. In addition, our method delivered high accuracy, precision, recall, and F1 scores in herniation localization, K-line status assessment, and T2 hyperintensity detection. Comparative analysis demonstrates that our system outperforms existing methods, establishing a new benchmark for segmentation and diagnostic tasks for cervical spondylosis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge