Jiangang Zou
A new method of modeling the multi-stage decision-making process of CRT using machine learning with uncertainty quantification
Sep 19, 2023
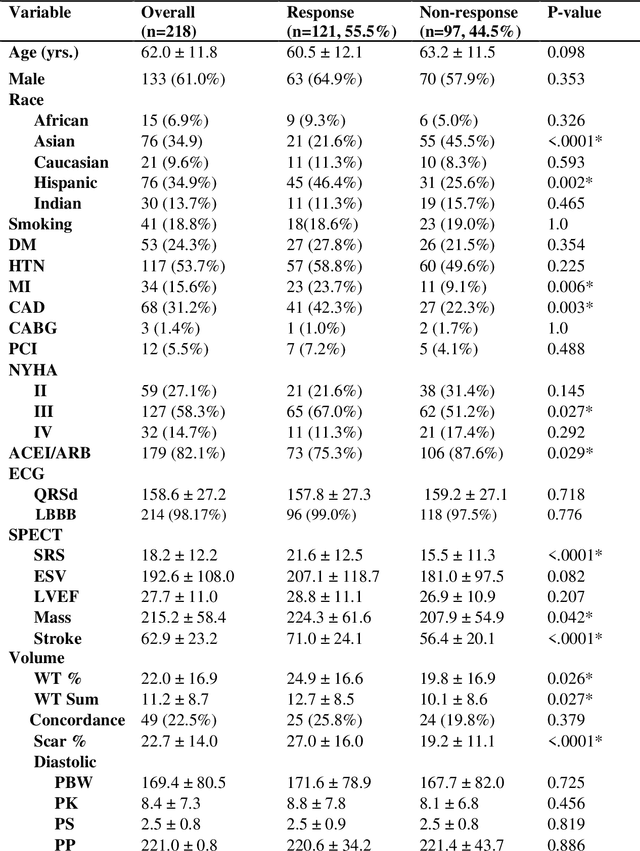
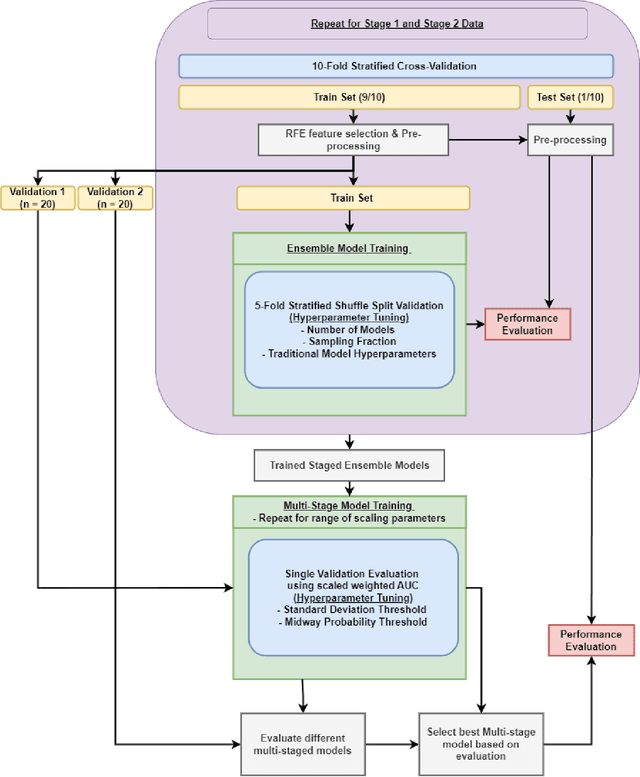
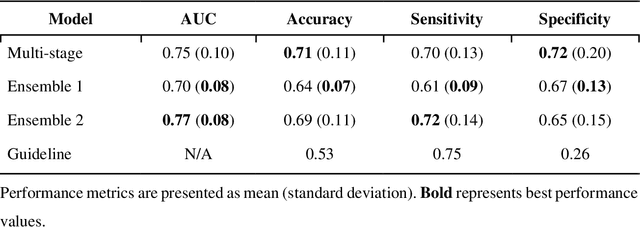
Abstract:Aims. The purpose of this study is to create a multi-stage machine learning model to predict cardiac resynchronization therapy (CRT) response for heart failure (HF) patients. This model exploits uncertainty quantification to recommend additional collection of single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI) variables if baseline clinical variables and features from electrocardiogram (ECG) are not sufficient. Methods. 218 patients who underwent rest-gated SPECT MPI were enrolled in this study. CRT response was defined as an increase in left ventricular ejection fraction (LVEF) > 5% at a 6 month follow-up. A multi-stage ML model was created by combining two ensemble models. Results. The response rate for CRT was 55.5% (n = 121) with overall male gender 61.0% (n = 133), an average age of 62.0, and LVEF of 27.7. The multi-stage model performed similarly to Ensemble 2 (which utilized the additional SPECT data) with AUC of 0.75 vs. 0.77, accuracy of 0.71 vs. 0.69, sensitivity of 0.70 vs. 0.72, and specificity 0.72 vs. 0.65, respectively. However, the multi-stage model only required SPECT MPI data for 52.7% of the patients across all folds. Conclusions. By using rule-based logic stemming from uncertainty quantification, the multi-stage model was able to reduce the need for additional SPECT MPI data acquisition without sacrificing performance.
A new method using deep transfer learning on ECG to predict the response to cardiac resynchronization therapy
Jun 02, 2023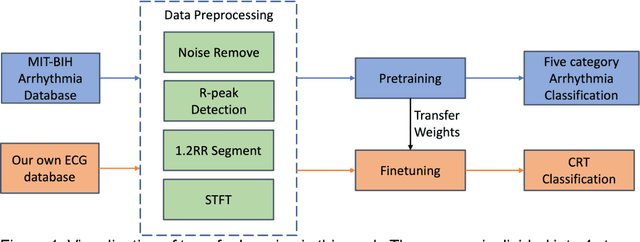
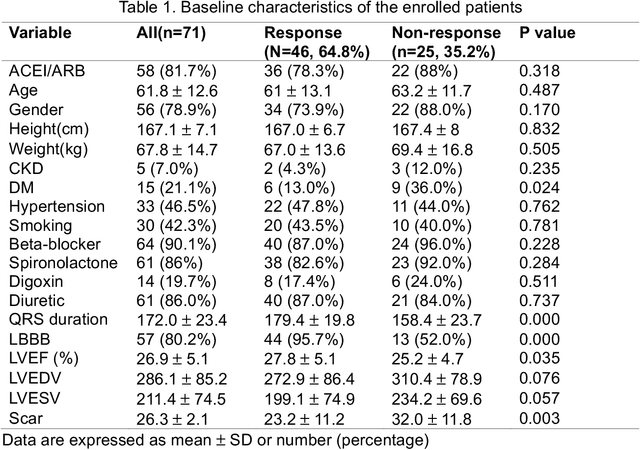
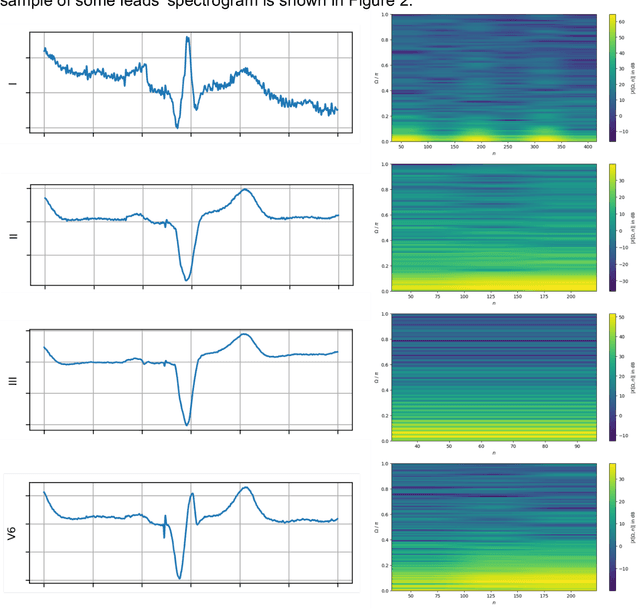
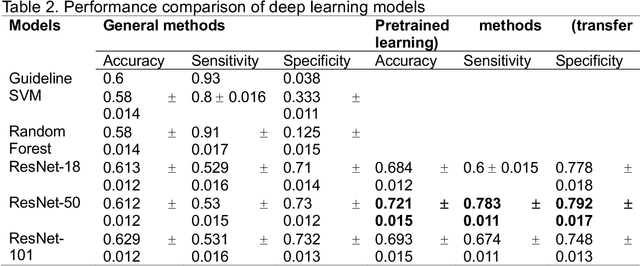
Abstract:Background: Cardiac resynchronization therapy (CRT) has emerged as an effective treatment for heart failure patients with electrical dyssynchrony. However, accurately predicting which patients will respond to CRT remains a challenge. This study explores the application of deep transfer learning techniques to train a predictive model for CRT response. Methods: In this study, the short-time Fourier transform (STFT) technique was employed to transform ECG signals into two-dimensional images. A transfer learning approach was then applied on the MIT-BIT ECG database to pre-train a convolutional neural network (CNN) model. The model was fine-tuned to extract relevant features from the ECG images, and then tested on our dataset of CRT patients to predict their response. Results: Seventy-one CRT patients were enrolled in this study. The transfer learning model achieved an accuracy of 72% in distinguishing responders from non-responders in the local dataset. Furthermore, the model showed good sensitivity (0.78) and specificity (0.79) in identifying CRT responders. The performance of our model outperformed clinic guidelines and traditional machine learning approaches. Conclusion: The utilization of ECG images as input and leveraging the power of transfer learning allows for improved accuracy in identifying CRT responders. This approach offers potential for enhancing patient selection and improving outcomes of CRT.
A new method using deep learning to predict the response to cardiac resynchronization therapy
May 04, 2023



Abstract:Background. Clinical parameters measured from gated single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI) have value in predicting cardiac resynchronization therapy (CRT) patient outcomes, but still show limitations. The purpose of this study is to combine clinical variables, features from electrocardiogram (ECG), and parameters from assessment of cardiac function with polarmaps from gated SPECT MPI through deep learning (DL) to predict CRT response. Methods. 218 patients who underwent rest gated SPECT MPI were enrolled in this study. CRT response was defined as an increase in left ventricular ejection fraction (LVEF) > 5% at a 6-month follow up. A DL model was constructed by combining a pre-trained VGG16 module and a multilayer perceptron. Two modalities of data were input to the model: polarmap images from SPECT MPI and tabular data from clinical features and ECG parameters. Gradient-weighted Class Activation Mapping (Grad-CAM) was applied to the VGG16 module to provide explainability for the polarmaps. For comparison, four machine learning (ML) models were trained using only the tabular features. Results. Modeling was performed on 218 patients who underwent CRT implantation with a response rate of 55.5% (n = 121). The DL model demonstrated average AUC (0.83), accuracy (0.73), sensitivity (0.76), and specificity (0.69) surpassing the ML models and guideline criteria. Guideline recommendations presented accuracy (0.53), sensitivity (0.75), and specificity (0.26). Conclusions. The DL model outperformed the ML models, showcasing the additional predictive benefit of utilizing SPECT MPI polarmaps. Incorporating additional patient data directly in the form of medical imagery can improve CRT response prediction.
A method using deep learning to discover new predictors of CRT response from mechanical dyssynchrony on gated SPECT MPI
Jun 01, 2021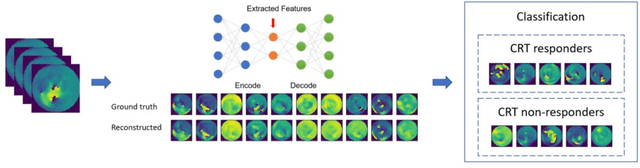
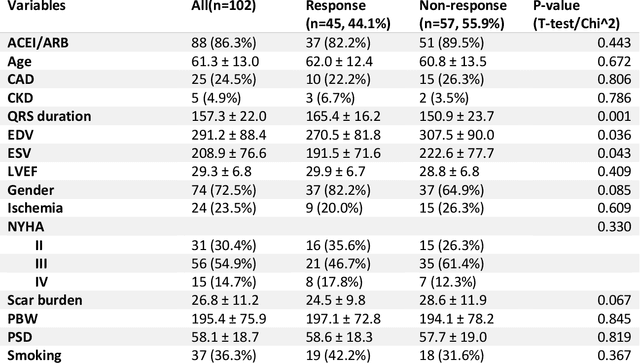
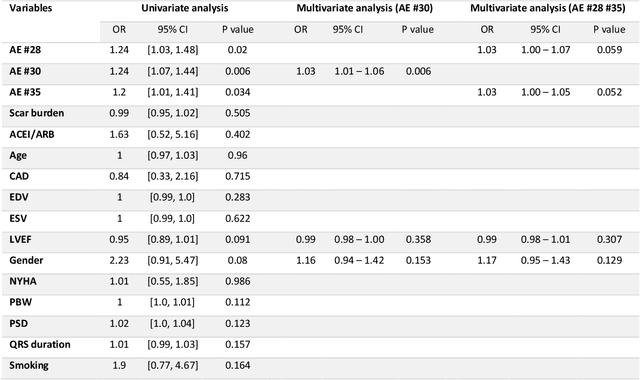
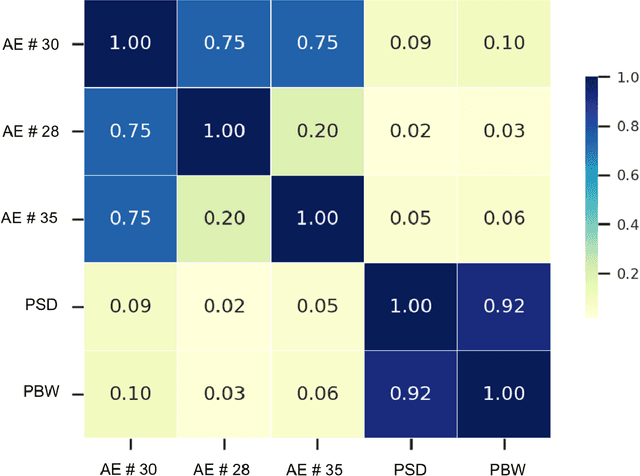
Abstract:Background. Studies have shown that the conventional left ventricular mechanical dyssynchrony (LVMD) parameters have their own statistical limitations. The purpose of this study is to extract new LVMD parameters from the phase analysis of gated SPECT MPI by deep learning to help CRT patient selection. Methods. One hundred and three patients who underwent rest gated SPECT MPI were enrolled in this study. CRT response was defined as a decrease in left ventricular end-systolic volume (LVESV) >= 15% at 6 +- 1 month follow up. Autoencoder (AE), an unsupervised deep learning method, was trained by the raw LV systolic phase polar maps to extract new LVMD parameters, called AE-based LVMD parameters. Correlation analysis was used to explain the relationships between new parameters with conventional LVMD parameters. Univariate and multivariate analyses were used to establish a multivariate model for predicting CRT response. Results. Complete data were obtained in 102 patients, 44.1% of them were classified as CRT responders. AE-based LVMD parameter was significant in the univariate (OR 1.24, 95% CI 1.07 - 1.44, P = 0.006) and multivariate analyses (OR 1.03, 95% CI 1.01 - 1.06, P = 0.006). Moreover, it had incremental value over PSD (AUC 0.72 vs. 0.63, LH 8.06, P = 0.005) and PBW (AUC 0.72 vs. 0.64, LH 7.87, P = 0.005), combined with significant clinic characteristics, including LVEF and gender. Conclusions. The new LVMD parameters extracted by autoencoder from the baseline gated SPECT MPI has the potential to improve the prediction of CRT response.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge