Jennifer A. Steeden
MultiFlow: A unified deep learning framework for multi-vessel classification, segmentation and clustering of phase-contrast MRI validated on a multi-site single ventricle patient cohort
Feb 17, 2025



Abstract:This study presents a unified deep learning (DL) framework, MultiFlowSeg, for classification and segmentation of velocity-encoded phase-contrast magnetic resonance imaging data, and MultiFlowDTC for temporal clustering of flow phenotypes. Applied to the FORCE registry of Fontan procedure patients, MultiFlowSeg achieved 100% classification accuracy for the aorta, SVC, and IVC, and 94% for the LPA and RPA. It demonstrated robust segmentation with a median Dice score of 0.91 (IQR: 0.86-0.93). The automated pipeline processed registry data, achieving high segmentation success despite challenges like poor image quality and dextrocardia. Temporal clustering identified five distinct patient subgroups, with significant differences in clinical outcomes, including ejection fraction, exercise tolerance, liver disease, and mortality. These results demonstrate the potential of combining DL and time-varying flow data for improved CHD prognosis and personalized care.
Deep Learning Pipeline for Preprocessing and Segmenting Cardiac Magnetic Resonance of Single Ventricle Patients from an Image Registry
Mar 21, 2023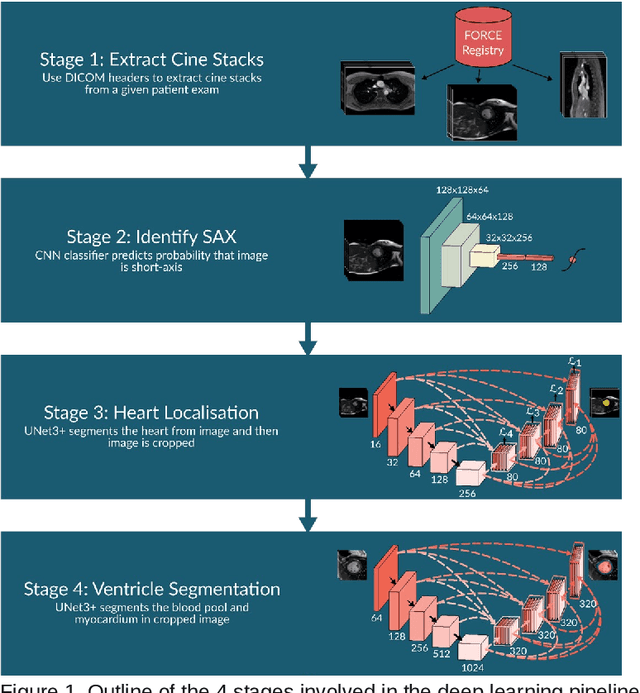
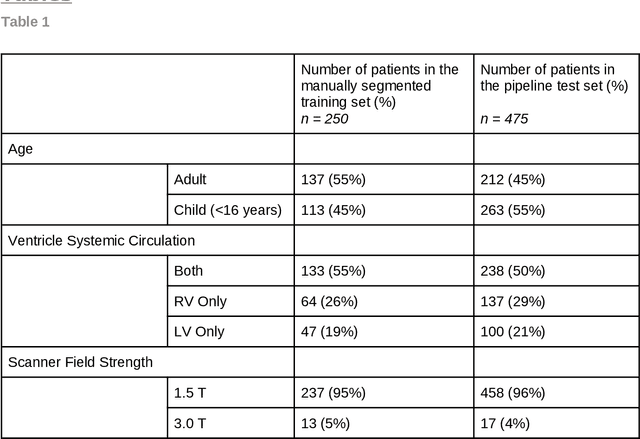
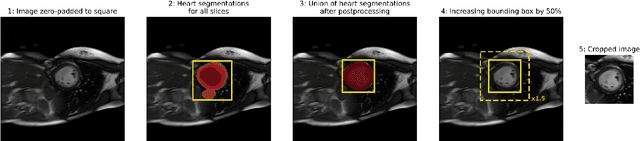
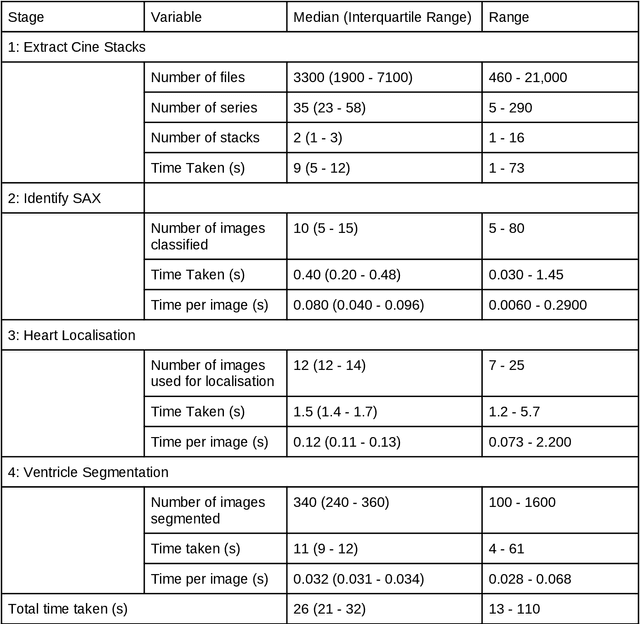
Abstract:Purpose: To develop and evaluate an end-to-end deep learning pipeline for segmentation and analysis of cardiac magnetic resonance images to provide core-lab processing for a multi-centre registry of Fontan patients. Materials and Methods: This retrospective study used training (n = 175), validation (n = 25) and testing (n = 50) cardiac magnetic resonance image exams collected from 13 institutions in the UK, US and Canada. The data was used to train and evaluate a pipeline containing three deep-learning models. The pipeline's performance was assessed on the Dice and IoU score between the automated and reference standard manual segmentation. Cardiac function values were calculated from both the automated and manual segmentation and evaluated using Bland-Altman analysis and paired t-tests. The overall pipeline was further evaluated qualitatively on 475 unseen patient exams. Results: For the 50 testing dataset, the pipeline achieved a median Dice score of 0.91 (0.89-0.94) for end-diastolic volume, 0.86 (0.82-0.89) for end-systolic volume, and 0.74 (0.70-0.77) for myocardial mass. The deep learning-derived end-diastolic volume, end-systolic volume, myocardial mass, stroke volume and ejection fraction had no statistical difference compared to the same values derived from manual segmentation with p values all greater than 0.05. For the 475 unseen patient exams, the pipeline achieved 68% adequate segmentation in both systole and diastole, 26% needed minor adjustments in either systole or diastole, 5% needed major adjustments, and the cropping model only failed in 0.4%. Conclusion: Deep learning pipeline can provide standardised 'core-lab' segmentation for Fontan patients. This pipeline can now be applied to the >4500 cardiac magnetic resonance exams currently in the FORCE registry as well as any new patients that are recruited.
Rapid Whole-Heart CMR with Single Volume Super-resolution
Dec 22, 2019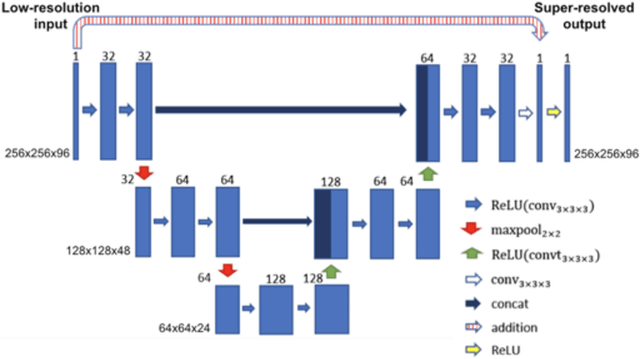
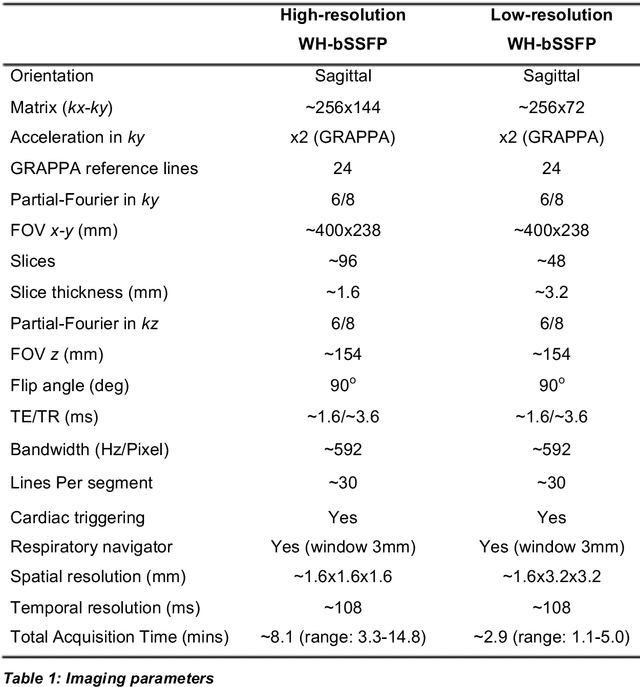
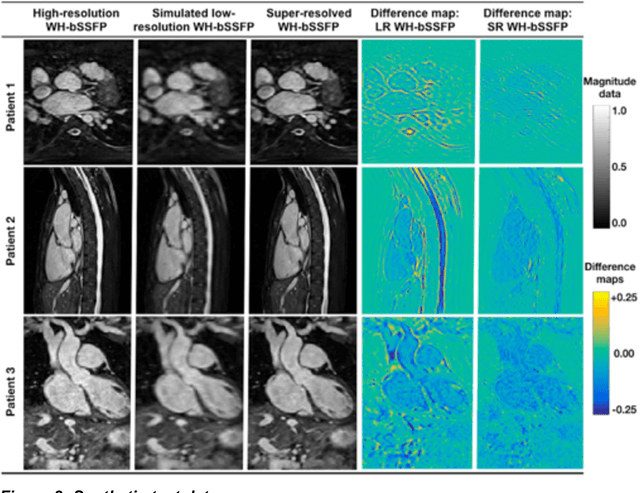
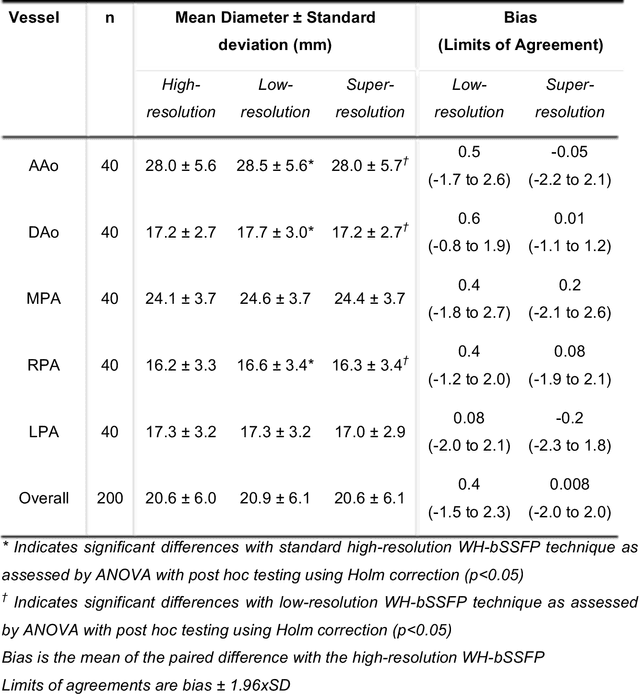
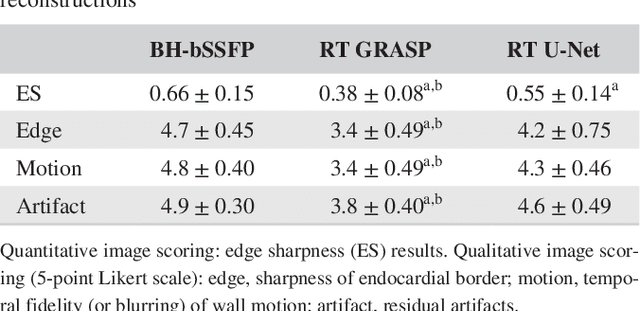
Abstract:Background: Three-dimensional, whole heart, balanced steady state free precession (WH-bSSFP) sequences provide delineation of intra-cardiac and vascular anatomy. However, they have long acquisition times. Here, we propose significant speed ups using a deep learning single volume super resolution reconstruction, to recover high resolution features from rapidly acquired low resolution WH-bSSFP images. Methods: A 3D residual U-Net was trained using synthetic data, created from a library of high-resolution WH-bSSFP images by simulating 0.5 slice resolution and 0.5 phase resolution. The trained network was validated with synthetic test data, as well as prospective low-resolution data. Results: Synthetic low-resolution data had significantly better image quality after super-resolution reconstruction. Qualitative image scores showed super-resolved images had better edge sharpness, fewer residual artefacts and less image distortion than low-resolution images, with similar scores to high-resolution data. Quantitative image scores showed super-resolved images had significantly better edge sharpness than low-resolution or high-resolution images, with significantly better signal-to-noise ratio than high-resolution data. Vessel diameters measurements showed over-estimation in the low-resolution measurements, compared to the high-resolution data. No significant differences and no bias was found in the super-resolution measurements. Conclusion: This paper demonstrates the potential of using a residual U-Net for super-resolution reconstruction of rapidly acquired low-resolution whole heart bSSFP data within a clinical setting. The resulting network can be applied very quickly, making these techniques particularly appealing within busy clinical workflow. Thus, we believe that this technique may help speed up whole heart CMR in clinical practice.
Real-time Cardiovascular MR with Spatio-temporal Artifact Suppression using Deep Learning - Proof of Concept in Congenital Heart Disease
Jun 14, 2018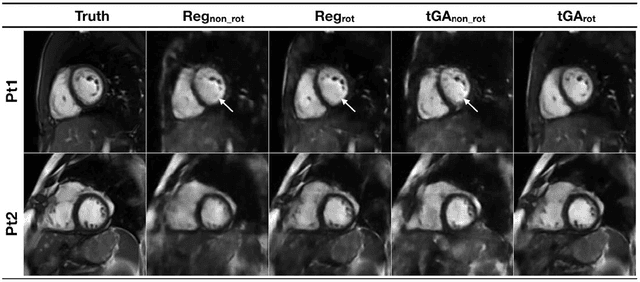
Abstract:PURPOSE: Real-time assessment of ventricular volumes requires high acceleration factors. Residual convolutional neural networks (CNN) have shown potential for removing artifacts caused by data undersampling. In this study we investigated the effect of different radial sampling patterns on the accuracy of a CNN. We also acquired actual real-time undersampled radial data in patients with congenital heart disease (CHD), and compare CNN reconstruction to Compressed Sensing (CS). METHODS: A 3D (2D plus time) CNN architecture was developed, and trained using 2276 gold-standard paired 3D data sets, with 14x radial undersampling. Four sampling schemes were tested, using 169 previously unseen 3D 'synthetic' test data sets. Actual real-time tiny Golden Angle (tGA) radial SSFP data was acquired in 10 new patients (122 3D data sets), and reconstructed using the 3D CNN as well as a CS algorithm; GRASP. RESULTS: Sampling pattern was shown to be important for image quality, and accurate visualisation of cardiac structures. For actual real-time data, overall reconstruction time with CNN (including creation of aliased images) was shown to be more than 5x faster than GRASP. Additionally, CNN image quality and accuracy of biventricular volumes was observed to be superior to GRASP for the same raw data. CONCLUSION: This paper has demonstrated the potential for the use of a 3D CNN for deep de-aliasing of real-time radial data, within the clinical setting. Clinical measures of ventricular volumes using real-time data with CNN reconstruction are not statistically significantly different from the gold-standard, cardiac gated, BH techniques.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge