Ioannis Sechopoulos
FlowNet-PET: Unsupervised Learning to Perform Respiratory Motion Correction in PET Imaging
May 27, 2022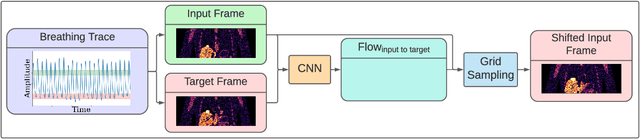
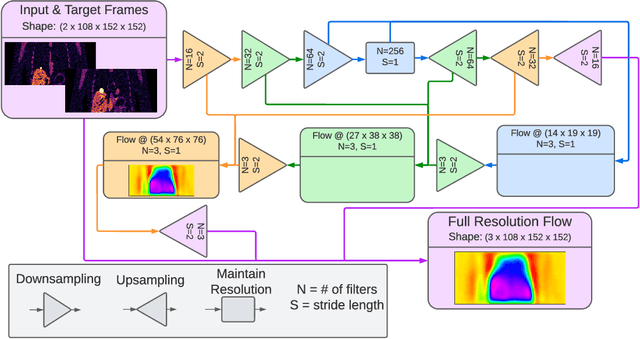
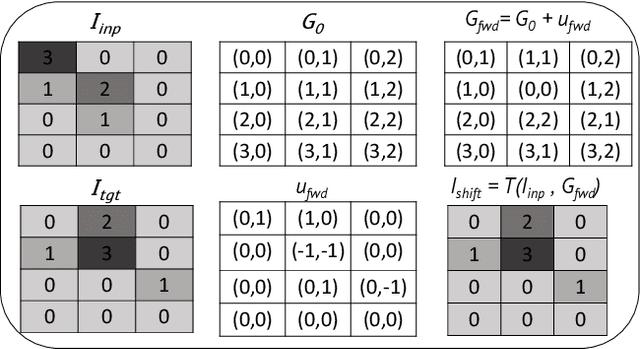
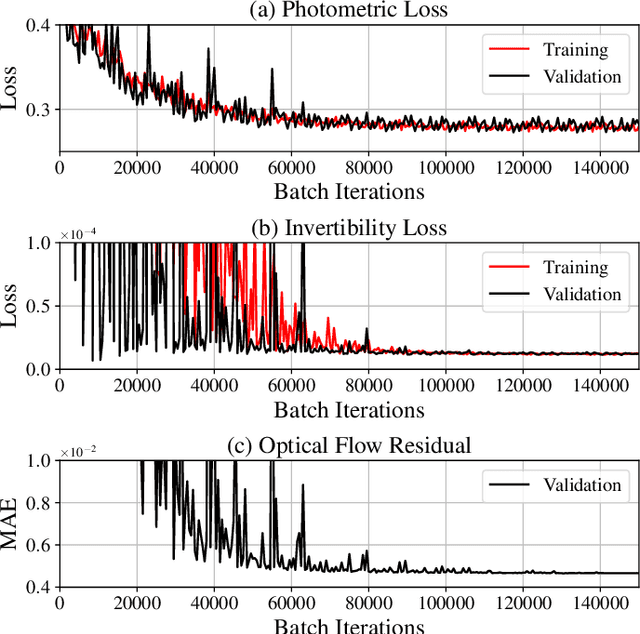
Abstract:To correct for breathing motion in PET imaging, an interpretable and unsupervised deep learning technique, FlowNet-PET, was constructed. The network was trained to predict the optical flow between two PET frames from different breathing amplitude ranges. As a result, the trained model groups different retrospectively-gated PET images together into a motion-corrected single bin, providing a final image with similar counting statistics as a non-gated image, but without the blurring effects that were initially observed. As a proof-of-concept, FlowNet-PET was applied to anthropomorphic digital phantom data, which provided the possibility to design robust metrics to quantify the corrections. When comparing the predicted optical flows to the ground truths, the median absolute error was found to be smaller than the pixel and slice widths, even for the phantom with a diaphragm movement of 21 mm. The improvements were illustrated by comparing against images without motion and computing the intersection over union (IoU) of the tumors as well as the enclosed activity and coefficient of variation (CoV) within the no-motion tumor volume before and after the corrections were applied. The average relative improvements provided by the network were 54%, 90%, and 76% for the IoU, total activity, and CoV, respectively. The results were then compared against the conventional retrospective phase binning approach. FlowNet-PET achieved similar results as retrospective binning, but only required one sixth of the scan duration. The code and data used for training and analysis has been made publicly available (https://github.com/teaghan/FlowNet_PET).
Deep learning reconstruction of digital breast tomosynthesis images for accurate breast density and patient-specific radiation dose estimation
Jun 11, 2020



Abstract:The two-dimensional nature of mammography makes estimation of the overall breast density challenging, and estimation of the true patient-specific radiation dose impossible. Digital breast tomosynthesis (DBT), a pseudo-3D technique, is now commonly used in breast cancer screening and diagnostics. Still, the severely limited 3rd dimension information in DBT has not been used, until now, to estimate the true breast density or the patient-specific dose. In this study, we propose a reconstruction algorithm for DBT based on deep learning specifically optimized for these tasks. The algorithm, which we name DBToR, is based on unrolling a proximal primal-dual optimization method, where the proximal operators are replaced with convolutional neural networks and prior knowledge is included in the model. This extends previous work on a deep learning based reconstruction model by providing both the primal and the dual blocks with breast thickness information, which is available in DBT. Training and testing of the model were performed using virtual patient phantoms from two different sources. Reconstruction performance, as well as accuracy in estimation of breast density and radiation dose, was estimated, showing high accuracy (density density < +/-3%; dose < +/-20%), without bias, significantly improving on the current state-of-the-art. This work also lays the groundwork for developing a deep learning-based reconstruction algorithm for the task of image interpretation by radiologists.
Deep Learning Framework for Digital Breast Tomosynthesis Reconstruction
Aug 14, 2018Abstract:Digital breast tomosynthesis is rapidly replacing digital mammography as the basic x-ray technique for evaluation of the breasts. However, the sparse sampling and limited angular range gives rise to different artifacts, which manufacturers try to solve in several ways. In this study we propose an extension of the Learned Primal-Dual algorithm for digital breast tomosynthesis. The Learned Primal-Dual algorithm is a deep neural network consisting of several `reconstruction blocks', which take in raw sinogram data as the initial input, perform a forward and a backward pass by taking projections and back-projections, and use a convolutional neural network to produce an intermediate reconstruction result which is then improved further by the successive reconstruction block. We extend the architecture by providing breast thickness measurements as a mask to the neural network and allow it to learn how to use this thickness mask. We have trained the algorithm on digital phantoms and the corresponding noise-free/noisy projections, and then tested the algorithm on digital phantoms for varying level of noise. Reconstruction performance of the algorithms was compared visually, using MSE loss and Structural Similarity Index. Results indicate that the proposed algorithm outperforms the baseline iterative reconstruction algorithm in terms of reconstruction quality for both breast edges and internal structures and is robust to noise.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge