Iman Beheshti
Decoding Cognitive Health Using Machine Learning: A Comprehensive Evaluation for Diagnosis of Significant Memory Concern
May 11, 2024Abstract:The timely identification of significant memory concern (SMC) is crucial for proactive cognitive health management, especially in an aging population. Detecting SMC early enables timely intervention and personalized care, potentially slowing cognitive disorder progression. This study presents a state-of-the-art review followed by a comprehensive evaluation of machine learning models within the randomized neural networks (RNNs) and hyperplane-based classifiers (HbCs) family to investigate SMC diagnosis thoroughly. Utilizing the Alzheimer's Disease Neuroimaging Initiative 2 (ADNI2) dataset, 111 individuals with SMC and 111 healthy older adults are analyzed based on T1W magnetic resonance imaging (MRI) scans, extracting rich features. This analysis is based on baseline structural MRI (sMRI) scans, extracting rich features from gray matter (GM), white matter (WM), Jacobian determinant (JD), and cortical thickness (CT) measurements. In RNNs, deep random vector functional link (dRVFL) and ensemble dRVFL (edRVFL) emerge as the best classifiers in terms of performance metrics in the identification of SMC. In HbCs, Kernelized pinball general twin support vector machine (Pin-GTSVM-K) excels in CT and WM features, whereas Linear Pin-GTSVM (Pin-GTSVM-L) and Linear intuitionistic fuzzy TSVM (IFTSVM-L) performs well in the JD and GM features sets, respectively. This comprehensive evaluation emphasizes the critical role of feature selection and model choice in attaining an effective classifier for SMC diagnosis. The inclusion of statistical analyses further reinforces the credibility of the results, affirming the rigor of this analysis. The performance measures exhibit the suitability of this framework in aiding researchers with the automated and accurate assessment of SMC. The source codes of the algorithms and datasets used in this study are available at https://github.com/mtanveer1/SMC.
Sex-based Disparities in Brain Aging: A Focus on Parkinson's Disease
Sep 18, 2023Abstract:PD is linked to faster brain aging. Sex is recognized as an important factor in PD, such that males are twice as likely as females to have the disease and have more severe symptoms and a faster progression rate. Despite previous research, there remains a significant gap in understanding the function of sex in the process of brain aging in PD patients. The T1-weighted MRI-driven brain-predicted age difference was computed in a group of 373 PD patients from the PPMI database using a robust brain-age estimation framework that was trained on 949 healthy subjects. Linear regression models were used to investigate the association between brain-PAD and clinical variables in PD, stratified by sex. All female PD patients were used in the correlational analysis while the same number of males were selected based on propensity score matching method considering age, education level, age of symptom onset, and clinical symptom severity. Despite both patient groups being matched for demographics, motor and non-motor symptoms, it was observed that males with Parkinson's disease exhibited a significantly higher mean brain age-delta than their female counterparts . In the propensity score-matched PD male group, brain-PAD was found to be associated with a decline in general cognition, a worse degree of sleep behavior disorder, reduced visuospatial acuity, and caudate atrophy. Conversely, no significant links were observed between these factors and brain-PAD in the PD female group.
Deep Learning for Brain Age Estimation: A Systematic Review
Dec 07, 2022Abstract:Over the years, Machine Learning models have been successfully employed on neuroimaging data for accurately predicting brain age. Deviations from the healthy brain aging pattern are associated to the accelerated brain aging and brain abnormalities. Hence, efficient and accurate diagnosis techniques are required for eliciting accurate brain age estimations. Several contributions have been reported in the past for this purpose, resorting to different data-driven modeling methods. Recently, deep neural networks (also referred to as deep learning) have become prevalent in manifold neuroimaging studies, including brain age estimation. In this review, we offer a comprehensive analysis of the literature related to the adoption of deep learning for brain age estimation with neuroimaging data. We detail and analyze different deep learning architectures used for this application, pausing at research works published to date quantitatively exploring their application. We also examine different brain age estimation frameworks, comparatively exposing their advantages and weaknesses. Finally, the review concludes with an outlook towards future directions that should be followed by prospective studies. The ultimate goal of this paper is to establish a common and informed reference for newcomers and experienced researchers willing to approach brain age estimation by using deep learning models
Diagnosis of Schizophrenia: A comprehensive evaluation
Mar 22, 2022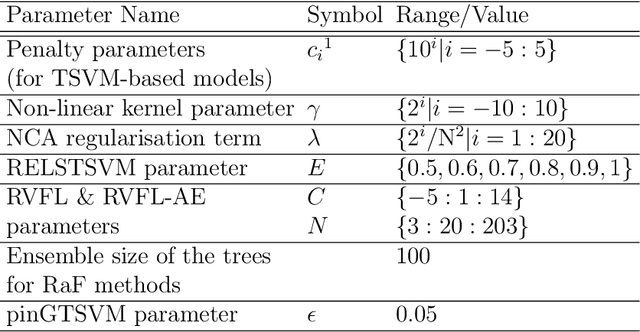
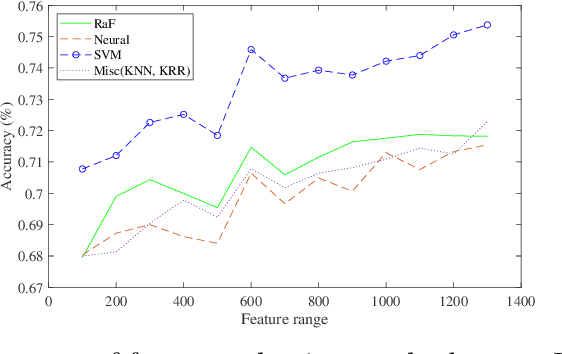
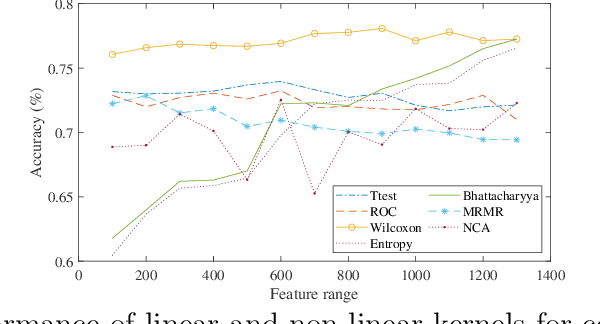
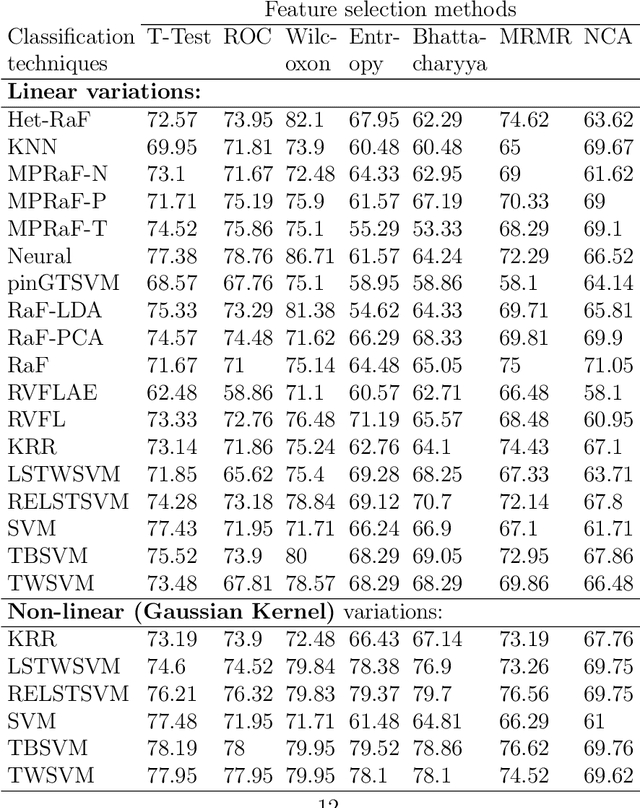
Abstract:Machine learning models have been successfully employed in the diagnosis of Schizophrenia disease. The impact of classification models and the feature selection techniques on the diagnosis of Schizophrenia have not been evaluated. Here, we sought to access the performance of classification models along with different feature selection approaches on the structural magnetic resonance imaging data. The data consist of 72 subjects with Schizophrenia and 74 healthy control subjects. We evaluated different classification algorithms based on support vector machine (SVM), random forest, kernel ridge regression and randomized neural networks. Moreover, we evaluated T-Test, Receiver Operator Characteristics (ROC), Wilcoxon, entropy, Bhattacharyya, Minimum Redundancy Maximum Relevance (MRMR) and Neighbourhood Component Analysis (NCA) as the feature selection techniques. Based on the evaluation, SVM based models with Gaussian kernel proved better compared to other classification models and Wilcoxon feature selection emerged as the best feature selection approach. Moreover, in terms of data modality the performance on integration of the grey matter and white matter proved better compared to the performance on the grey and white matter individually. Our evaluation showed that classification algorithms along with the feature selection approaches impact the diagnosis of Schizophrenia disease. This indicates that proper selection of the features and the classification models can improve the diagnosis of Schizophrenia.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge