Iain Pierce
Imaging Transformer for MRI Denoising: a Scalable Model Architecture that enables SNR << 1 Imaging
Apr 13, 2025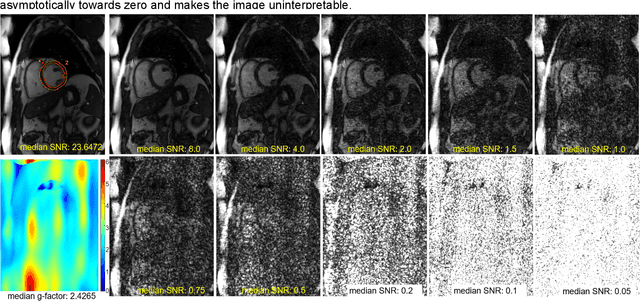

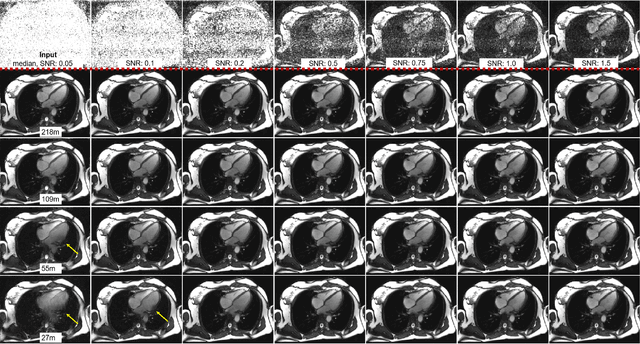
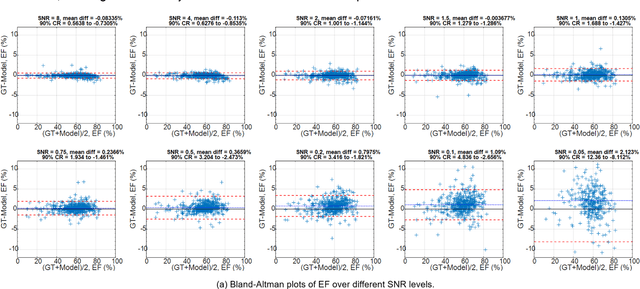
Abstract:Purpose: To propose a flexible and scalable imaging transformer (IT) architecture with three attention modules for multi-dimensional imaging data and apply it to MRI denoising with very low input SNR. Methods: Three independent attention modules were developed: spatial local, spatial global, and frame attentions. They capture long-range signal correlation and bring back the locality of information in images. An attention-cell-block design processes 5D tensors ([B, C, F, H, W]) for 2D, 2D+T, and 3D image data. A High Resolution (HRNet) backbone was built to hold IT blocks. Training dataset consists of 206,677 cine series and test datasets had 7,267 series. Ten input SNR levels from 0.05 to 8.0 were tested. IT models were compared to seven convolutional and transformer baselines. To test scalability, four IT models 27m to 218m parameters were trained. Two senior cardiologists reviewed IT model outputs from which the EF was measured and compared against the ground-truth. Results: IT models significantly outperformed other models over the tested SNR levels. The performance gap was most prominent at low SNR levels. The IT-218m model had the highest SSIM and PSNR, restoring good image quality and anatomical details even at SNR 0.2. Two experts agreed at this SNR or above, the IT model output gave the same clinical interpretation as the ground-truth. The model produced images that had accurate EF measurements compared to ground-truth values. Conclusions: Imaging transformer model offers strong performance, scalability, and versatility for MR denoising. It recovers image quality suitable for confident clinical reading and accurate EF measurement, even at very low input SNR of 0.2.
Inline AI: Open-source Deep Learning Inference for Cardiac MR
Apr 03, 2024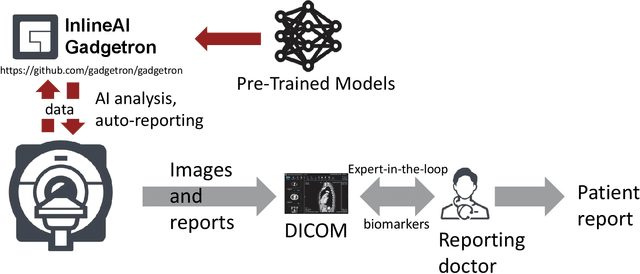
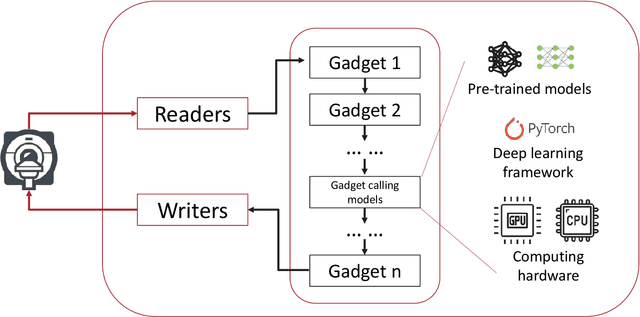
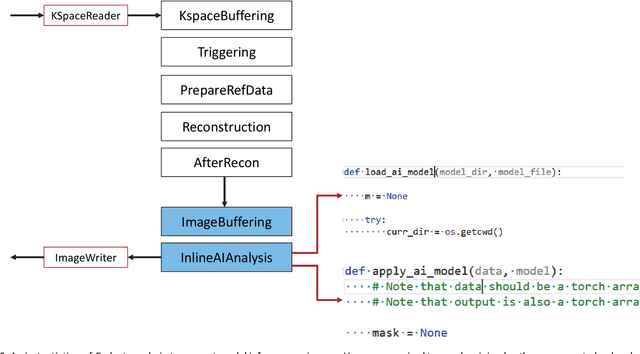
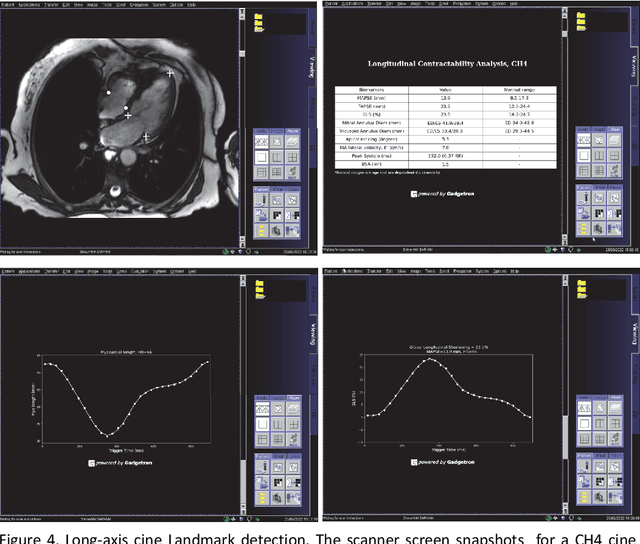
Abstract:Cardiac Magnetic Resonance (CMR) is established as a non-invasive imaging technique for evaluation of heart function, anatomy, and myocardial tissue characterization. Quantitative biomarkers are central for diagnosis and management of heart disease. Deep learning (DL) is playing an ever more important role in extracting these quantitative measures from CMR images. While many researchers have reported promising results in training and evaluating models, model deployment into the imaging workflow is less explored. A new imaging AI framework, the InlineAI, was developed and open-sourced. The main innovation is to enable the model inference inline as a part of imaging computation, instead of as an offline post-processing step and to allow users to plug in their models. We demonstrate the system capability on three applications: long-axis CMR cine landmark detection, short-axis CMR cine analysis of function and anatomy, and quantitative perfusion mapping. The InlineAI allowed models to be deployed into imaging workflow in a streaming manner directly on the scanner. The model was loaded and inference on incoming images were performed while the data acquisition was ongoing, and results were sent back to scanner. Several biomarkers were extracted from model outputs in the demonstrated applications and reported as curves and tabular values. All processes are full automated. the model inference was completed within 6-45s after the end of imaging data acquisition.
Imaging transformer for MRI denoising with the SNR unit training: enabling generalization across field-strengths, imaging contrasts, and anatomy
Apr 03, 2024

Abstract:The ability to recover MRI signal from noise is key to achieve fast acquisition, accurate quantification, and high image quality. Past work has shown convolutional neural networks can be used with abundant and paired low and high-SNR images for training. However, for applications where high-SNR data is difficult to produce at scale (e.g. with aggressive acceleration, high resolution, or low field strength), training a new denoising network using a large quantity of high-SNR images can be infeasible. In this study, we overcome this limitation by improving the generalization of denoising models, enabling application to many settings beyond what appears in the training data. Specifically, we a) develop a training scheme that uses complex MRIs reconstructed in the SNR units (i.e., the images have a fixed noise level, SNR unit training) and augments images with realistic noise based on coil g-factor, and b) develop a novel imaging transformer (imformer) to handle 2D, 2D+T, and 3D MRIs in one model architecture. Through empirical evaluation, we show this combination improves performance compared to CNN models and improves generalization, enabling a denoising model to be used across field-strengths, image contrasts, and anatomy.
Bayesian Uncertainty Estimation by Hamiltonian Monte Carlo: Applications to Cardiac MRI Segmentation
Mar 04, 2024Abstract:Deep learning (DL)-based methods have achieved state-of-the-art performance for a wide range of medical image segmentation tasks. Nevertheless, recent studies show that deep neural networks (DNNs) can be miscalibrated and overconfident, leading to "silent failures" that are risky} for clinical applications. Bayesian statistics provide an intuitive approach to DL failure detection, based on posterior probability estimation. However, Bayesian DL, and in particular the posterior estimation, is intractable for large medical image segmentation DNNs. To tackle this challenge, we propose a Bayesian learning framework by Hamiltonian Monte Carlo (HMC), tempered by cold posterior (CP) to accommodate medical data augmentation, named HMC-CP. For HMC computation, we further propose a cyclical annealing strategy, which captures both local and global geometries of the posterior distribution, enabling highly efficient Bayesian DNN training with the same computational budget requirements as training a single DNN. The resulting Bayesian DNN outputs an ensemble segmentation along with the segmentation uncertainty. We evaluate the proposed HMC-CP extensively on cardiac magnetic resonance image (MRI) segmentation, using in-domain steady-state free precession (SSFP) cine images as well as out-of-domain datasets of quantitative $T_1$ and $T_2$ mapping.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge