Hongzheng Yang
VP-Bench: A Comprehensive Benchmark for Visual Prompting in Multimodal Large Language Models
Nov 14, 2025Abstract:Multimodal large language models (MLLMs) have enabled a wide range of advanced vision-language applications, including fine-grained object recognition and contextual understanding. When querying specific regions or objects in an image, human users naturally use "visual prompts" (VPs), such as bounding boxes, to provide reference. However, no existing benchmark systematically evaluates the ability of MLLMs to interpret such VPs. This gap leaves it unclear whether current MLLMs can effectively recognize VPs, an intuitive prompting method for humans, and use them to solve problems. To address this limitation, we introduce VP-Bench, a benchmark for assessing MLLMs' capability in VP perception and utilization. VP-Bench employs a two-stage evaluation framework: Stage 1 examines models' ability to perceive VPs in natural scenes, using 30k visualized prompts spanning eight shapes and 355 attribute combinations. Stage 2 investigates the impact of VPs on downstream tasks, measuring their effectiveness in real-world problem-solving scenarios. Using VP-Bench, we evaluate 28 MLLMs, including proprietary systems (e.g., GPT-4o) and open-source models (e.g., InternVL3 and Qwen2.5-VL), and provide a comprehensive analysis of factors that affect VP understanding, such as variations in VP attributes, question arrangement, and model scale. VP-Bench establishes a new reference framework for studying how MLLMs comprehend and resolve grounded referring questions.
From Exploration to Exploitation: A Two-Stage Entropy RLVR Approach for Noise-Tolerant MLLM Training
Nov 11, 2025



Abstract:Reinforcement Learning with Verifiable Rewards (RLVR) for Multimodal Large Language Models (MLLMs) is highly dependent on high-quality labeled data, which is often scarce and prone to substantial annotation noise in real-world scenarios. Existing unsupervised RLVR methods, including pure entropy minimization, can overfit to incorrect labels and limit the crucial reward ranking signal for Group-Relative Policy Optimization (GRPO). To address these challenges and enhance noise tolerance, we propose a novel two-stage, token-level entropy optimization method for RLVR. This approach dynamically guides the model from exploration to exploitation during training. In the initial exploration phase, token-level entropy maximization promotes diverse and stochastic output generation, serving as a strong regularizer that prevents premature convergence to noisy labels and ensures sufficient intra-group variation, which enables more reliable reward gradient estimation in GRPO. As training progresses, the method transitions into the exploitation phase, where token-level entropy minimization encourages the model to produce confident and deterministic outputs, thereby consolidating acquired knowledge and refining prediction accuracy. Empirically, across three MLLM backbones - Qwen2-VL-2B, Qwen2-VL-7B, and Qwen2.5-VL-3B - spanning diverse noise settings and multiple tasks, our phased strategy consistently outperforms prior approaches by unifying and enhancing external, internal, and entropy-based methods, delivering robust and superior performance across the board.
Better Reasoning with Less Data: Enhancing VLMs Through Unified Modality Scoring
Jun 10, 2025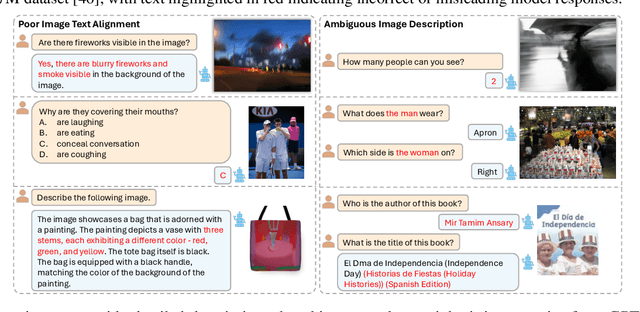
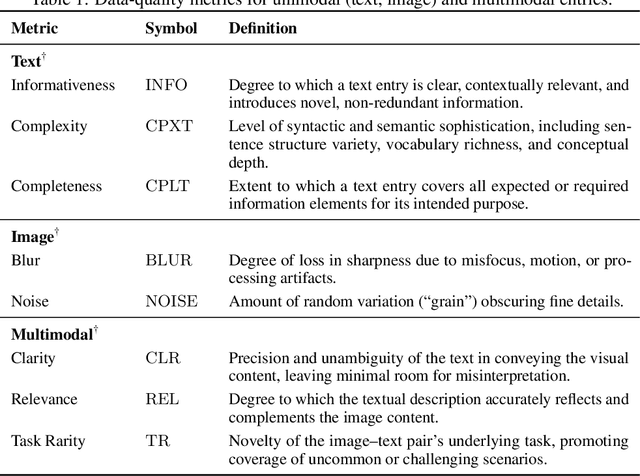
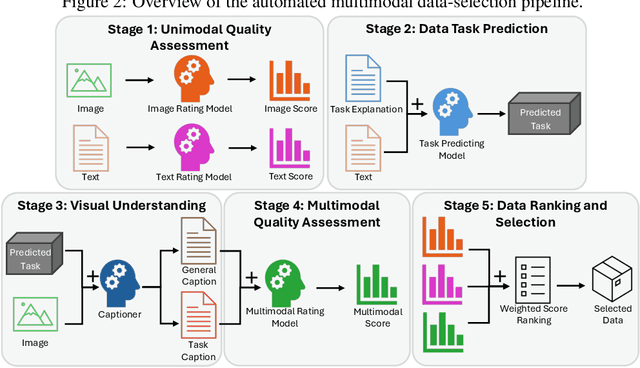
Abstract:The application of visual instruction tuning and other post-training techniques has significantly enhanced the capabilities of Large Language Models (LLMs) in visual understanding, enriching Vision-Language Models (VLMs) with more comprehensive visual language datasets. However, the effectiveness of VLMs is highly dependent on large-scale, high-quality datasets that ensure precise recognition and accurate reasoning. Two key challenges hinder progress: (1) noisy alignments between images and the corresponding text, which leads to misinterpretation, and (2) ambiguous or misleading text, which obscures visual content. To address these challenges, we propose SCALE (Single modality data quality and Cross modality Alignment Evaluation), a novel quality-driven data selection pipeline for VLM instruction tuning datasets. Specifically, SCALE integrates a cross-modality assessment framework that first assigns each data entry to its appropriate vision-language task, generates general and task-specific captions (covering scenes, objects, style, etc.), and evaluates the alignment, clarity, task rarity, text coherence, and image clarity of each entry based on the generated captions. We reveal that: (1) current unimodal quality assessment methods evaluate one modality while overlooking the rest, which can underestimate samples essential for specific tasks and discard the lower-quality instances that help build model robustness; and (2) appropriately generated image captions provide an efficient way to transfer the image-text multimodal task into a unified text modality.
Does Representation Intervention Really Identify Desired Concepts and Elicit Alignment?
May 24, 2025Abstract:Representation intervention aims to locate and modify the representations that encode the underlying concepts in Large Language Models (LLMs) to elicit the aligned and expected behaviors. Despite the empirical success, it has never been examined whether one could locate the faithful concepts for intervention. In this work, we explore the question in safety alignment. If the interventions are faithful, the intervened LLMs should erase the harmful concepts and be robust to both in-distribution adversarial prompts and the out-of-distribution (OOD) jailbreaks. While it is feasible to erase harmful concepts without degrading the benign functionalities of LLMs in linear settings, we show that it is infeasible in the general non-linear setting. To tackle the issue, we propose Concept Concentration (COCA). Instead of identifying the faithful locations to intervene, COCA refractors the training data with an explicit reasoning process, which firstly identifies the potential unsafe concepts and then decides the responses. Essentially, COCA simplifies the decision boundary between harmful and benign representations, enabling more effective linear erasure. Extensive experiments with multiple representation intervention methods and model architectures demonstrate that COCA significantly reduces both in-distribution and OOD jailbreak success rates, and meanwhile maintaining strong performance on regular tasks such as math and code generation.
SEFE: Superficial and Essential Forgetting Eliminator for Multimodal Continual Instruction Tuning
May 05, 2025Abstract:Multimodal Continual Instruction Tuning (MCIT) aims to enable Multimodal Large Language Models (MLLMs) to incrementally learn new tasks without catastrophic forgetting. In this paper, we explore forgetting in this context, categorizing it into superficial forgetting and essential forgetting. Superficial forgetting refers to cases where the model's knowledge may not be genuinely lost, but its responses to previous tasks deviate from expected formats due to the influence of subsequent tasks' answer styles, making the results unusable. By contrast, essential forgetting refers to situations where the model provides correctly formatted but factually inaccurate answers, indicating a true loss of knowledge. Assessing essential forgetting necessitates addressing superficial forgetting first, as severe superficial forgetting can obscure the model's knowledge state. Hence, we first introduce the Answer Style Diversification (ASD) paradigm, which defines a standardized process for transforming data styles across different tasks, unifying their training sets into similarly diversified styles to prevent superficial forgetting caused by style shifts. Building on this, we propose RegLoRA to mitigate essential forgetting. RegLoRA stabilizes key parameters where prior knowledge is primarily stored by applying regularization, enabling the model to retain existing competencies. Experimental results demonstrate that our overall method, SEFE, achieves state-of-the-art performance.
Uncertainty Estimation for Safety-critical Scene Segmentation via Fine-grained Reward Maximization
Nov 05, 2023



Abstract:Uncertainty estimation plays an important role for future reliable deployment of deep segmentation models in safety-critical scenarios such as medical applications. However, existing methods for uncertainty estimation have been limited by the lack of explicit guidance for calibrating the prediction risk and model confidence. In this work, we propose a novel fine-grained reward maximization (FGRM) framework, to address uncertainty estimation by directly utilizing an uncertainty metric related reward function with a reinforcement learning based model tuning algorithm. This would benefit the model uncertainty estimation through direct optimization guidance for model calibration. Specifically, our method designs a new uncertainty estimation reward function using the calibration metric, which is maximized to fine-tune an evidential learning pre-trained segmentation model for calibrating prediction risk. Importantly, we innovate an effective fine-grained parameter update scheme, which imposes fine-grained reward-weighting of each network parameter according to the parameter importance quantified by the fisher information matrix. To the best of our knowledge, this is the first work exploring reward optimization for model uncertainty estimation in safety-critical vision tasks. The effectiveness of our method is demonstrated on two large safety-critical surgical scene segmentation datasets under two different uncertainty estimation settings. With real-time one forward pass at inference, our method outperforms state-of-the-art methods by a clear margin on all the calibration metrics of uncertainty estimation, while maintaining a high task accuracy for the segmentation results. Code is available at \url{https://github.com/med-air/FGRM}.
Dynamic Bank Learning for Semi-supervised Federated Image Diagnosis with Class Imbalance
Jun 27, 2022


Abstract:Despite recent progress on semi-supervised federated learning (FL) for medical image diagnosis, the problem of imbalanced class distributions among unlabeled clients is still unsolved for real-world use. In this paper, we study a practical yet challenging problem of class imbalanced semi-supervised FL (imFed-Semi), which allows all clients to have only unlabeled data while the server just has a small amount of labeled data. This imFed-Semi problem is addressed by a novel dynamic bank learning scheme, which improves client training by exploiting class proportion information. This scheme consists of two parts, i.e., the dynamic bank construction to distill various class proportions for each local client, and the sub-bank classification to impose the local model to learn different class proportions. We evaluate our approach on two public real-world medical datasets, including the intracranial hemorrhage diagnosis with 25,000 CT slices and skin lesion diagnosis with 10,015 dermoscopy images. The effectiveness of our method has been validated with significant performance improvements (7.61% and 4.69%) compared with the second-best on the accuracy, as well as comprehensive analytical studies. Code is available at https://github.com/med-air/imFedSemi.
DLTTA: Dynamic Learning Rate for Test-time Adaptation on Cross-domain Medical Images
May 27, 2022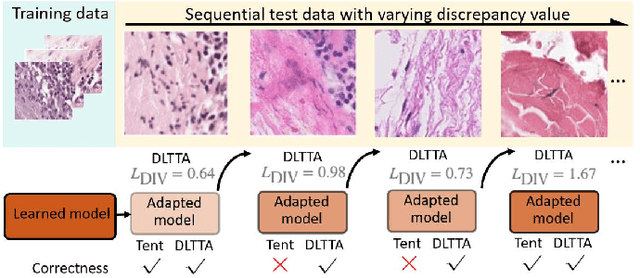
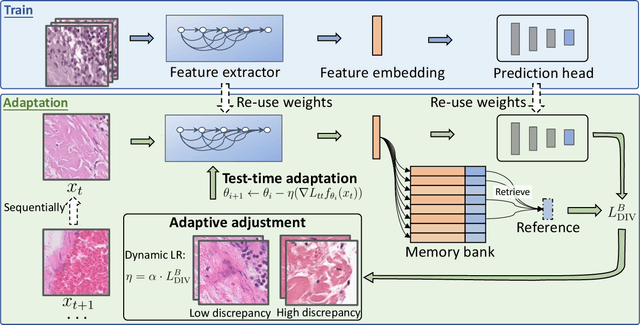
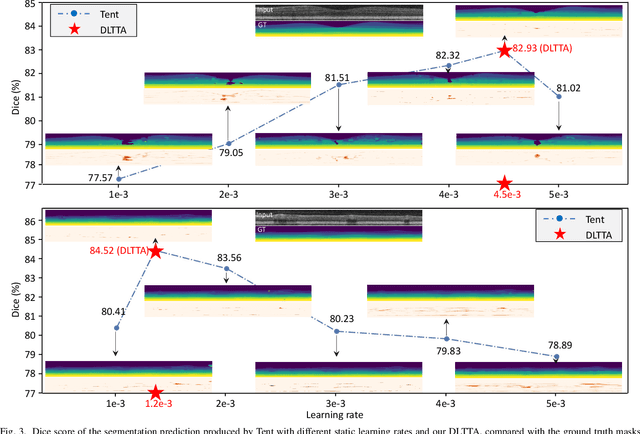
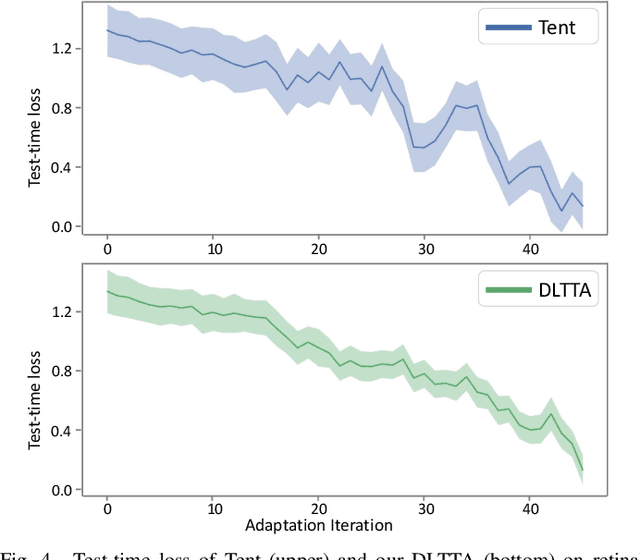
Abstract:Test-time adaptation (TTA) has increasingly been an important topic to efficiently tackle the cross-domain distribution shift at test time for medical images from different institutions. Previous TTA methods have a common limitation of using a fixed learning rate for all the test samples. Such a practice would be sub-optimal for TTA, because test data may arrive sequentially therefore the scale of distribution shift would change frequently. To address this problem, we propose a novel dynamic learning rate adjustment method for test-time adaptation, called DLTTA, which dynamically modulates the amount of weights update for each test image to account for the differences in their distribution shift. Specifically, our DLTTA is equipped with a memory bank based estimation scheme to effectively measure the discrepancy of a given test sample. Based on this estimated discrepancy, a dynamic learning rate adjustment strategy is then developed to achieve a suitable degree of adaptation for each test sample. The effectiveness and general applicability of our DLTTA is extensively demonstrated on three tasks including retinal optical coherence tomography (OCT) segmentation, histopathological image classification, and prostate 3D MRI segmentation. Our method achieves effective and fast test-time adaptation with consistent performance improvement over current state-of-the-art test-time adaptation methods. Code is available at: https://github.com/med-air/DLTTA.
IOP-FL: Inside-Outside Personalization for Federated Medical Image Segmentation
Apr 16, 2022



Abstract:Federated learning (FL) allows multiple medical institutions to collaboratively learn a global model without centralizing all clients data. It is difficult, if possible at all, for such a global model to commonly achieve optimal performance for each individual client, due to the heterogeneity of medical data from various scanners and patient demographics. This problem becomes even more significant when deploying the global model to unseen clients outside the FL with new distributions not presented during federated training. To optimize the prediction accuracy of each individual client for critical medical tasks, we propose a novel unified framework for both Inside and Outside model Personalization in FL (IOP-FL). Our inside personalization is achieved by a lightweight gradient-based approach that exploits the local adapted model for each client, by accumulating both the global gradients for common knowledge and local gradients for client-specific optimization. Moreover, and importantly, the obtained local personalized models and the global model can form a diverse and informative routing space to personalize a new model for outside FL clients. Hence, we design a new test-time routing scheme inspired by the consistency loss with a shape constraint to dynamically incorporate the models, given the distribution information conveyed by the test data. Our extensive experimental results on two medical image segmentation tasks present significant improvements over SOTA methods on both inside and outside personalization, demonstrating the great potential of our IOP-FL scheme for clinical practice. Code will be released at https://github.com/med-air/IOP-FL.
Federated Semi-supervised Medical Image Classification via Inter-client Relation Matching
Jun 16, 2021


Abstract:Federated learning (FL) has emerged with increasing popularity to collaborate distributed medical institutions for training deep networks. However, despite existing FL algorithms only allow the supervised training setting, most hospitals in realistic usually cannot afford the intricate data labeling due to absence of budget or expertise. This paper studies a practical yet challenging FL problem, named \textit{Federated Semi-supervised Learning} (FSSL), which aims to learn a federated model by jointly utilizing the data from both labeled and unlabeled clients (i.e., hospitals). We present a novel approach for this problem, which improves over traditional consistency regularization mechanism with a new inter-client relation matching scheme. The proposed learning scheme explicitly connects the learning across labeled and unlabeled clients by aligning their extracted disease relationships, thereby mitigating the deficiency of task knowledge at unlabeled clients and promoting discriminative information from unlabeled samples. We validate our method on two large-scale medical image classification datasets. The effectiveness of our method has been demonstrated with the clear improvements over state-of-the-arts as well as the thorough ablation analysis on both tasks\footnote{Code will be made available at \url{https://github.com/liuquande/FedIRM}}.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge