Ezgi Mercan
Classifying Breast Histopathology Images with a Ductal Instance-Oriented Pipeline
Dec 11, 2020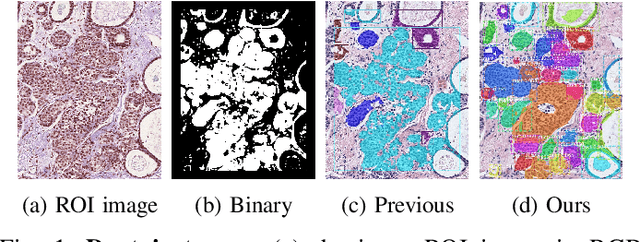
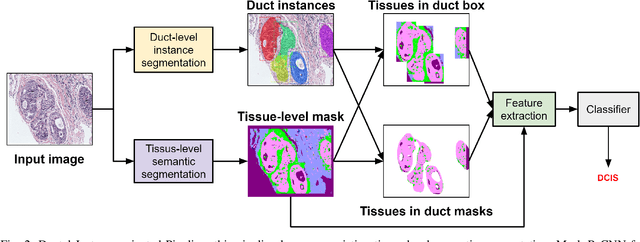
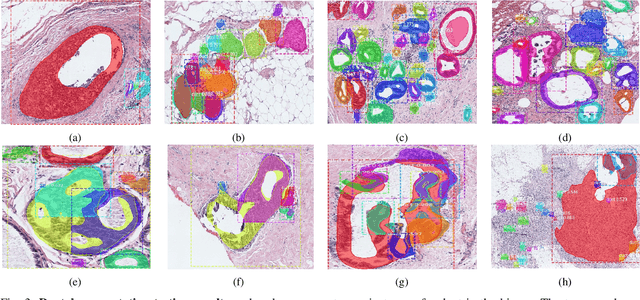
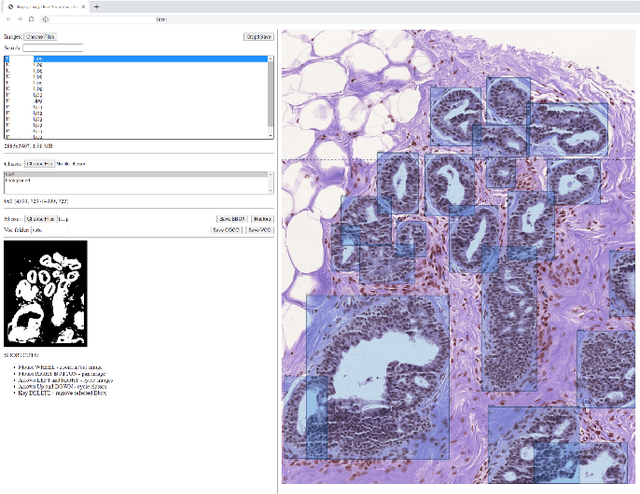
Abstract:In this study, we propose the Ductal Instance-Oriented Pipeline (DIOP) that contains a duct-level instance segmentation model, a tissue-level semantic segmentation model, and three-levels of features for diagnostic classification. Based on recent advancements in instance segmentation and the Mask R-CNN model, our duct-level segmenter tries to identify each ductal individual inside a microscopic image; then, it extracts tissue-level information from the identified ductal instances. Leveraging three levels of information obtained from these ductal instances and also the histopathology image, the proposed DIOP outperforms previous approaches (both feature-based and CNN-based) in all diagnostic tasks; for the four-way classification task, the DIOP achieves comparable performance to general pathologists in this unique dataset. The proposed DIOP only takes a few seconds to run in the inference time, which could be used interactively on most modern computers. More clinical explorations are needed to study the robustness and generalizability of this system in the future.
Y-Net: Joint Segmentation and Classification for Diagnosis of Breast Biopsy Images
Jun 04, 2018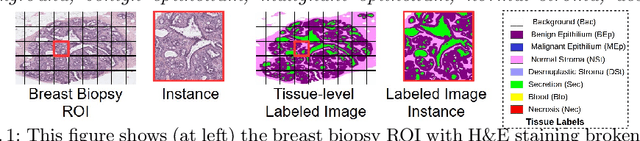
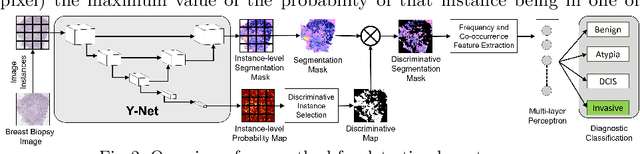
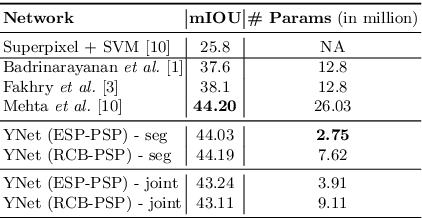
Abstract:In this paper, we introduce a conceptually simple network for generating discriminative tissue-level segmentation masks for the purpose of breast cancer diagnosis. Our method efficiently segments different types of tissues in breast biopsy images while simultaneously predicting a discriminative map for identifying important areas in an image. Our network, Y-Net, extends and generalizes U-Net by adding a parallel branch for discriminative map generation and by supporting convolutional block modularity, which allows the user to adjust network efficiency without altering the network topology. Y-Net delivers state-of-the-art segmentation accuracy while learning 6.6x fewer parameters than its closest competitors. The addition of descriptive power from Y-Net's discriminative segmentation masks improve diagnostic classification accuracy by 7% over state-of-the-art methods for diagnostic classification. Source code is available at: https://sacmehta.github.io/YNet.
Learning to Segment Breast Biopsy Whole Slide Images
Oct 10, 2017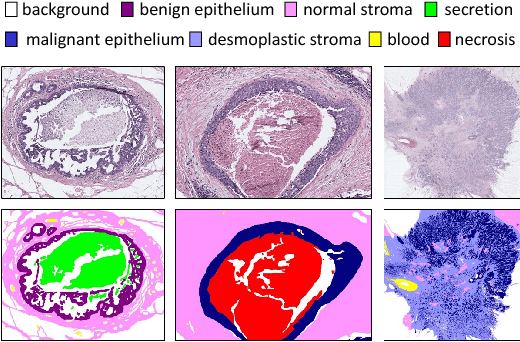
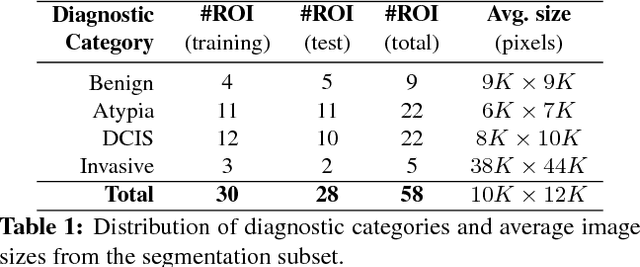
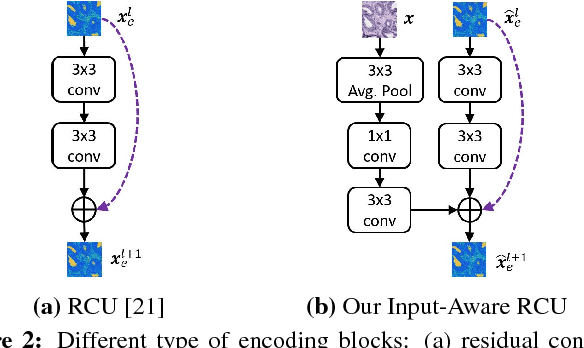
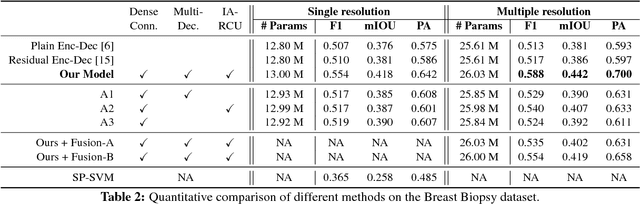
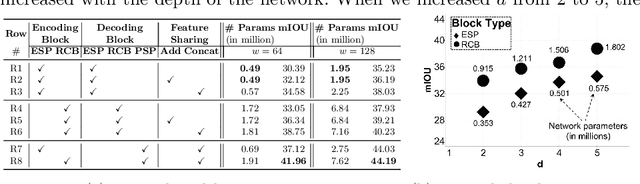
Abstract:We trained and applied an encoder-decoder model to semantically segment breast biopsy images into biologically meaningful tissue labels. Since conventional encoder-decoder networks cannot be applied directly on large biopsy images and the different sized structures in biopsies present novel challenges, we propose four modifications: (1) an input-aware encoding block to compensate for information loss, (2) a new dense connection pattern between encoder and decoder, (3) dense and sparse decoders to combine multi-level features, (4) a multi-resolution network that fuses the results of encoder-decoders run on different resolutions. Our model outperforms a feature-based approach and conventional encoder-decoders from the literature. We use semantic segmentations produced with our model in an automated diagnosis task and obtain higher accuracies than a baseline approach that employs an SVM for feature-based segmentation, both using the same segmentation-based diagnostic features.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge