Eswar Damaraju
Spatio-temporal Dynamics of Intrinsic Networks in Functional Magnetic Imaging Data Using Recurrent Neural Networks
Aug 27, 2018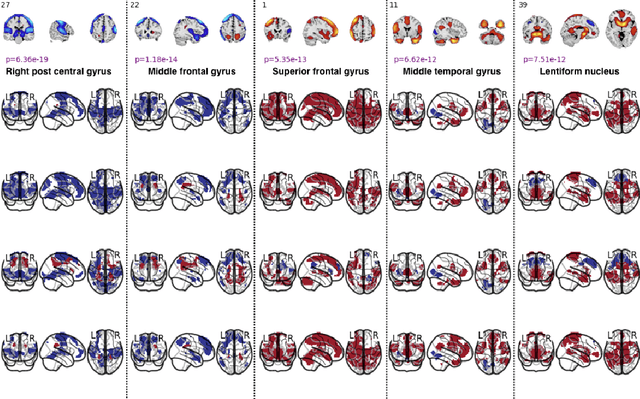
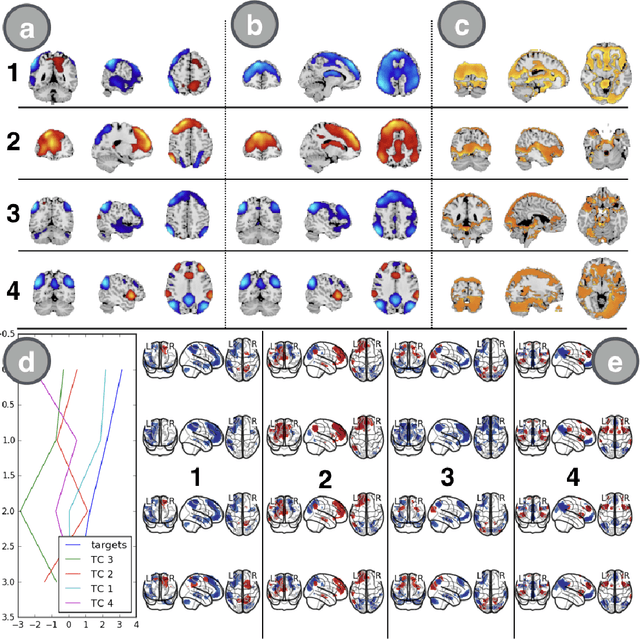
Abstract:We introduce a novel recurrent neural network (RNN) approach to account for temporal dynamics and dependencies in brain networks observed via functional magnetic resonance imaging (fMRI). Our approach directly parameterizes temporal dynamics through recurrent connections, which can be used to formulate blind source separation with a conditional (rather than marginal) independence assumption, which we call RNN-ICA. This formulation enables us to visualize the temporal dynamics of both first order (activity) and second order (directed connectivity) information in brain networks that are widely studied in a static sense, but not well-characterized dynamically. RNN-ICA predicts dynamics directly from the recurrent states of the RNN in both task and resting state fMRI. Our results show both task-related and group-differentiating directed connectivity.
Almost instant brain atlas segmentation for large-scale studies
Nov 01, 2017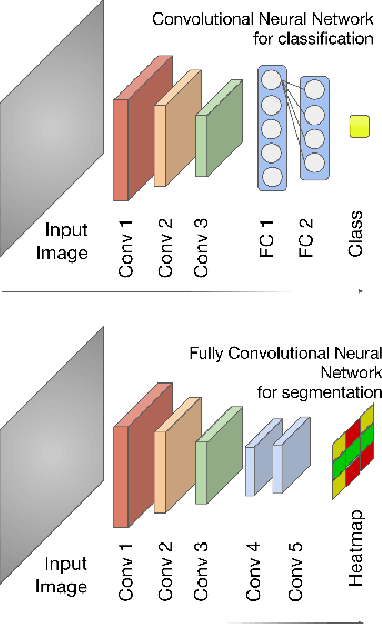
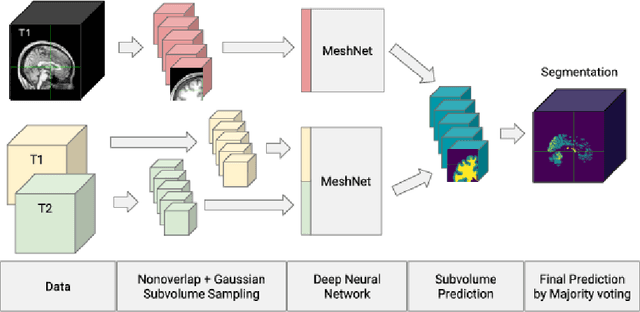
Abstract:Large scale studies of group differences in healthy controls and patients and screenings for early stage disease prevention programs require processing and analysis of extensive multisubject datasets. Complexity of the task increases even further when segmenting structural MRI of the brain into an atlas with more than 50 regions. Current automatic approaches are time-consuming and hardly scalable; they often involve many error prone intermediate steps and don't utilize other available modalities. To alleviate these problems, we propose a feedforward fully convolutional neural network trained on the output produced by the state of the art models. Incredible speed due to available powerful GPUs neural network makes this analysis much easier and faster (from $>10$ hours to a minute). The proposed model is more than two orders of magnitudes faster than the state of the art and yet as accurate. We have evaluated the network's performance by comparing it with the state of the art in the task of differentiating region volumes of healthy controls and patients with schizophrenia on a dataset with 311 subjects. This comparison provides a strong evidence that speed did not harm the accuracy. The overall quality may also be increased by utilizing multi-modal datasets (not an easy task for other models) by simple adding more modalities as an input. Our model will be useful in large-scale studies as well as in clinical care solutions, where it can significantly reduce delay between the patient screening and the result.
End-to-end learning of brain tissue segmentation from imperfect labeling
Jun 05, 2017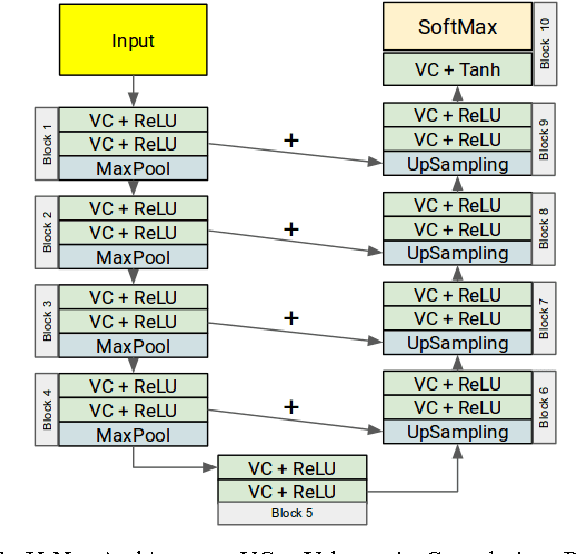
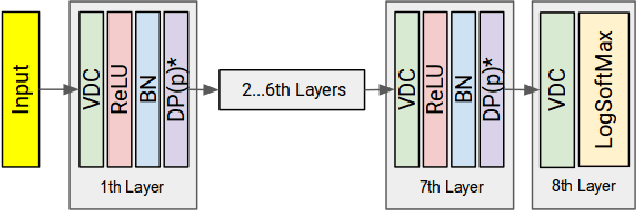
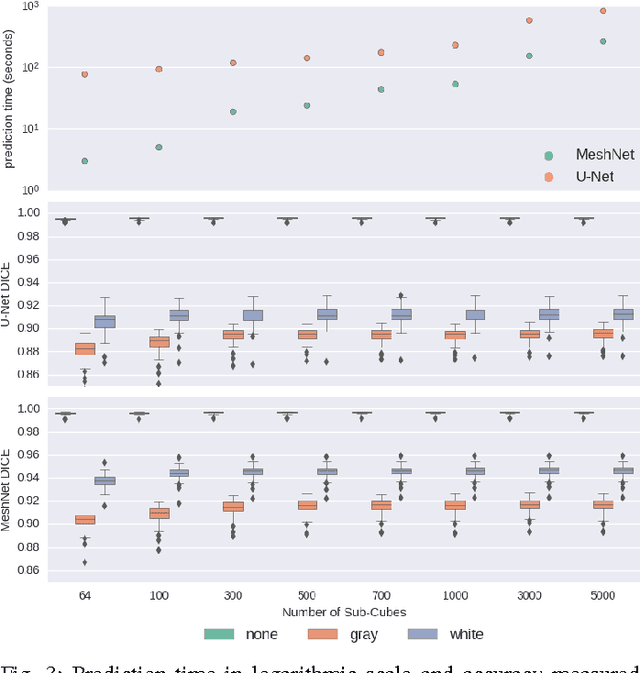
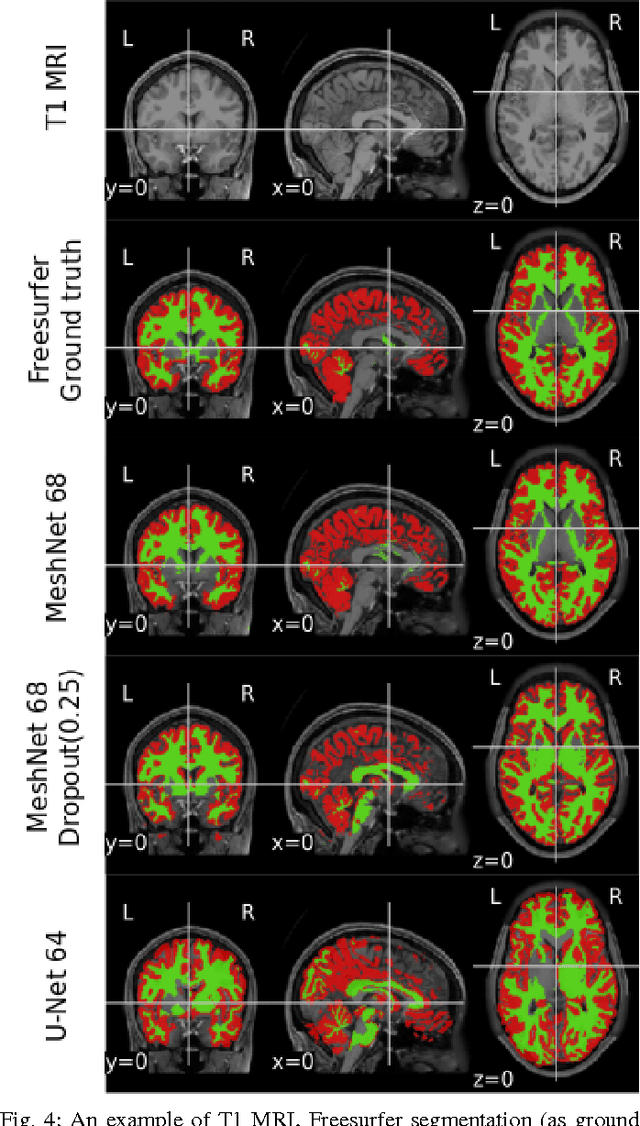
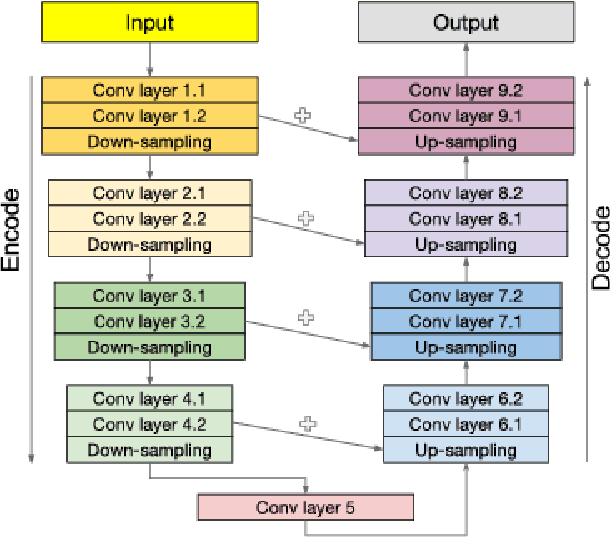
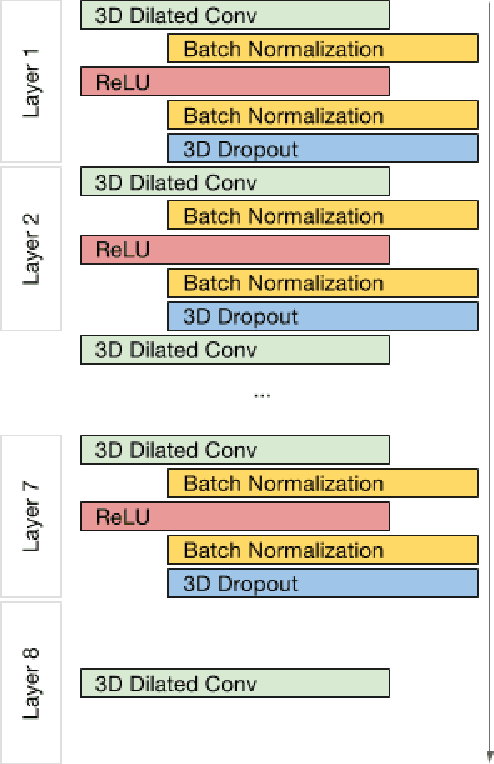
Abstract:Segmenting a structural magnetic resonance imaging (MRI) scan is an important pre-processing step for analytic procedures and subsequent inferences about longitudinal tissue changes. Manual segmentation defines the current gold standard in quality but is prohibitively expensive. Automatic approaches are computationally intensive, incredibly slow at scale, and error prone due to usually involving many potentially faulty intermediate steps. In order to streamline the segmentation, we introduce a deep learning model that is based on volumetric dilated convolutions, subsequently reducing both processing time and errors. Compared to its competitors, the model has a reduced set of parameters and thus is easier to train and much faster to execute. The contrast in performance between the dilated network and its competitors becomes obvious when both are tested on a large dataset of unprocessed human brain volumes. The dilated network consistently outperforms not only another state-of-the-art deep learning approach, the up convolutional network, but also the ground truth on which it was trained. Not only can the incredible speed of our model make large scale analyses much easier but we also believe it has great potential in a clinical setting where, with little to no substantial delay, a patient and provider can go over test results.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge