Dieuwertje Alblas
Geometric deep learning for local growth prediction on abdominal aortic aneurysm surfaces
Jun 11, 2025Abstract:Abdominal aortic aneurysms (AAAs) are progressive focal dilatations of the abdominal aorta. AAAs may rupture, with a survival rate of only 20\%. Current clinical guidelines recommend elective surgical repair when the maximum AAA diameter exceeds 55 mm in men or 50 mm in women. Patients that do not meet these criteria are periodically monitored, with surveillance intervals based on the maximum AAA diameter. However, this diameter does not take into account the complex relation between the 3D AAA shape and its growth, making standardized intervals potentially unfit. Personalized AAA growth predictions could improve monitoring strategies. We propose to use an SE(3)-symmetric transformer model to predict AAA growth directly on the vascular model surface enriched with local, multi-physical features. In contrast to other works which have parameterized the AAA shape, this representation preserves the vascular surface's anatomical structure and geometric fidelity. We train our model using a longitudinal dataset of 113 computed tomography angiography (CTA) scans of 24 AAA patients at irregularly sampled intervals. After training, our model predicts AAA growth to the next scan moment with a median diameter error of 1.18 mm. We further demonstrate our model's utility to identify whether a patient will become eligible for elective repair within two years (acc = 0.93). Finally, we evaluate our model's generalization on an external validation set consisting of 25 CTAs from 7 AAA patients from a different hospital. Our results show that local directional AAA growth prediction from the vascular surface is feasible and may contribute to personalized surveillance strategies.
Global Control for Local SO-Equivariant Scale-Invariant Vessel Segmentation
Mar 22, 2024



Abstract:Personalized 3D vascular models can aid in a range of diagnostic, prognostic, and treatment-planning tasks relevant to cardiovascular disease management. Deep learning provides a means to automatically obtain such models. Ideally, a user should have control over the exact region of interest (ROI) to be included in a vascular model, and the model should be watertight and highly accurate. To this end, we propose a combination of a global controller leveraging voxel mask segmentations to provide boundary conditions for vessels of interest to a local, iterative vessel segmentation model. We introduce the preservation of scale- and rotational symmetries in the local segmentation model, leading to generalisation to vessels of unseen sizes and orientations. Combined with the global controller, this enables flexible 3D vascular model building, without additional retraining. We demonstrate the potential of our method on a dataset containing abdominal aortic aneurysms (AAAs). Our method performs on par with a state-of-the-art segmentation model in the segmentation of AAAs, iliac arteries and renal arteries, while providing a watertight, smooth surface segmentation. Moreover, we demonstrate that by adapting the global controller, we can easily extend vessel sections in the 3D model.
SIRE: scale-invariant, rotation-equivariant estimation of artery orientations using graph neural networks
Nov 09, 2023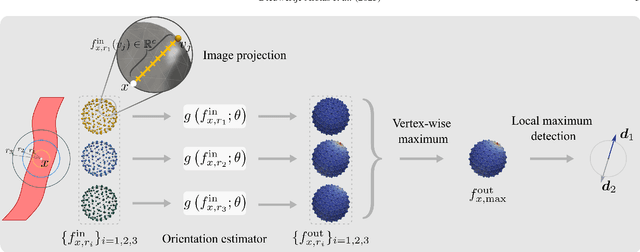
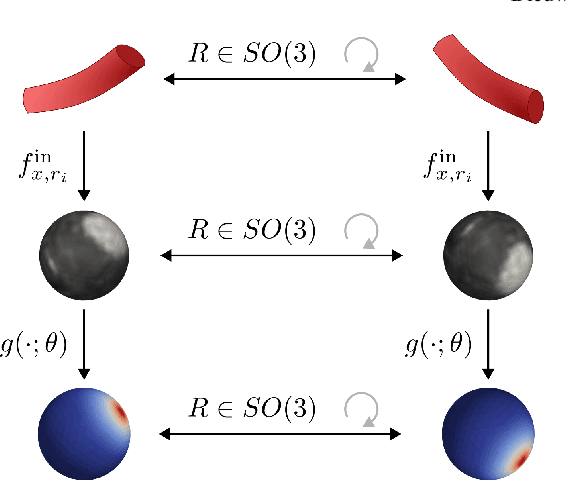
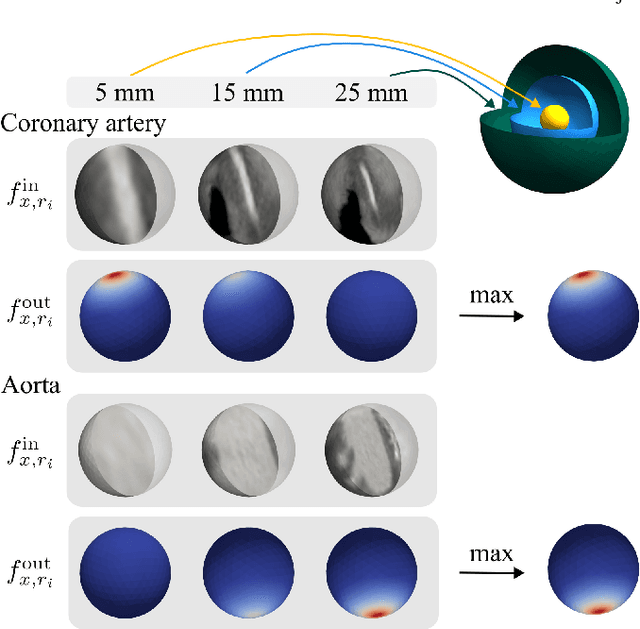
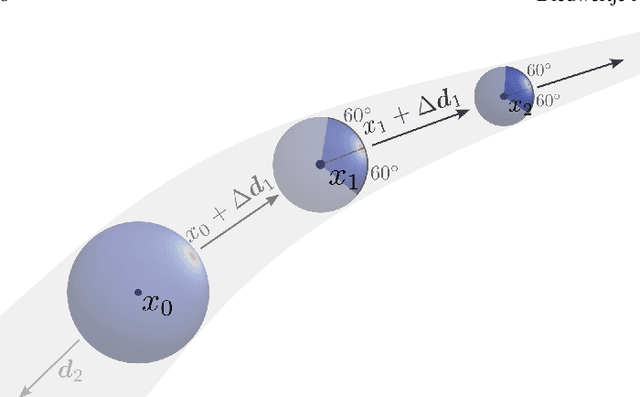
Abstract:Blood vessel orientation as visualized in 3D medical images is an important descriptor of its geometry that can be used for centerline extraction and subsequent segmentation and visualization. Arteries appear at many scales and levels of tortuosity, and determining their exact orientation is challenging. Recent works have used 3D convolutional neural networks (CNNs) for this purpose, but CNNs are sensitive to varying vessel sizes and orientations. We present SIRE: a scale-invariant, rotation-equivariant estimator for local vessel orientation. SIRE is modular and can generalise due to symmetry preservation. SIRE consists of a gauge equivariant mesh CNN (GEM-CNN) operating on multiple nested spherical meshes with different sizes in parallel. The features on each mesh are a projection of image intensities within the corresponding sphere. These features are intrinsic to the sphere and, in combination with the GEM-CNN, lead to SO(3)-equivariance. Approximate scale invariance is achieved by weight sharing and use of a symmetric maximum function to combine multi-scale predictions. Hence, SIRE can be trained with arbitrarily oriented vessels with varying radii to generalise to vessels with a wide range of calibres and tortuosity. We demonstrate the efficacy of SIRE using three datasets containing vessels of varying scales: the vascular model repository (VMR), the ASOCA coronary artery set, and a set of abdominal aortic aneurysms (AAAs). We embed SIRE in a centerline tracker which accurately tracks AAAs, regardless of the data SIRE is trained with. Moreover, SIRE can be used to track coronary arteries, even when trained only with AAAs. In conclusion, by incorporating SO(3) and scale symmetries, SIRE can determine the orientations of vessels outside of the training domain, forming a robust and data-efficient solution to geometric analysis of blood vessels in 3D medical images.
Uncertainty-based quality assurance of carotid artery wall segmentation in black-blood MRI
Aug 18, 2023Abstract:The application of deep learning models to large-scale data sets requires means for automatic quality assurance. We have previously developed a fully automatic algorithm for carotid artery wall segmentation in black-blood MRI that we aim to apply to large-scale data sets. This method identifies nested artery walls in 3D patches centered on the carotid artery. In this study, we investigate to what extent the uncertainty in the model predictions for the contour location can serve as a surrogate for error detection and, consequently, automatic quality assurance. We express the quality of automatic segmentations using the Dice similarity coefficient. The uncertainty in the model's prediction is estimated using either Monte Carlo dropout or test-time data augmentation. We found that (1) including uncertainty measurements did not degrade the quality of the segmentations, (2) uncertainty metrics provide a good proxy of the quality of our contours if the center found during the first step is enclosed in the lumen of the carotid artery and (3) they could be used to detect low-quality segmentations at the participant level. This automatic quality assurance tool might enable the application of our model in large-scale data sets.
Implicit Neural Representations for Modeling of Abdominal Aortic Aneurysm Progression
Mar 02, 2023



Abstract:Abdominal aortic aneurysms (AAAs) are progressive dilatations of the abdominal aorta that, if left untreated, can rupture with lethal consequences. Imaging-based patient monitoring is required to select patients eligible for surgical repair. In this work, we present a model based on implicit neural representations (INRs) to model AAA progression. We represent the AAA wall over time as the zero-level set of a signed distance function (SDF), estimated by a multilayer perception that operates on space and time. We optimize this INR using automatically extracted segmentation masks in longitudinal CT data. This network is conditioned on spatiotemporal coordinates and represents the AAA surface at any desired resolution at any moment in time. Using regularization on spatial and temporal gradients of the SDF, we ensure proper interpolation of the AAA shape. We demonstrate the network's ability to produce AAA interpolations with average surface distances ranging between 0.72 and 2.52 mm from images acquired at highly irregular intervals. The results indicate that our model can accurately interpolate AAA shapes over time, with potential clinical value for a more personalised assessment of AAA progression.
Going Off-Grid: Continuous Implicit Neural Representations for 3D Vascular Modeling
Jul 29, 2022



Abstract:Personalised 3D vascular models are valuable for diagnosis, prognosis and treatment planning in patients with cardiovascular disease. Traditionally, such models have been constructed with explicit representations such as meshes and voxel masks, or implicit representations such as radial basis functions or atomic (tubular) shapes. Here, we propose to represent surfaces by the zero level set of their signed distance function (SDF) in a differentiable implicit neural representation (INR). This allows us to model complex vascular structures with a representation that is implicit, continuous, light-weight, and easy to integrate with deep learning algorithms. We here demonstrate the potential of this approach with three practical examples. First, we obtain an accurate and watertight surface for an abdominal aortic aneurysm (AAA) from CT images and show robust fitting from as little as 200 points on the surface. Second, we simultaneously fit nested vessel walls in a single INR without intersections. Third, we show how 3D models of individual arteries can be smoothly blended into a single watertight surface. Our results show that INRs are a flexible representation with potential for minimally interactive annotation and manipulation of complex vascular structures.
Deep Learning-Based Carotid Artery Vessel Wall Segmentation in Black-Blood MRI Using Anatomical Priors
Dec 02, 2021



Abstract:Carotid artery vessel wall thickness measurement is an essential step in the monitoring of patients with atherosclerosis. This requires accurate segmentation of the vessel wall, i.e., the region between an artery's lumen and outer wall, in black-blood magnetic resonance (MR) images. Commonly used convolutional neural networks (CNNs) for semantic segmentation are suboptimal for this task as their use does not guarantee a contiguous ring-shaped segmentation. Instead, in this work, we cast vessel wall segmentation as a multi-task regression problem in a polar coordinate system. For each carotid artery in each axial image slice, we aim to simultaneously find two non-intersecting nested contours that together delineate the vessel wall. CNNs applied to this problem enable an inductive bias that guarantees ring-shaped vessel walls. Moreover, we identify a problem-specific training data augmentation technique that substantially affects segmentation performance. We apply our method to segmentation of the internal and external carotid artery wall, and achieve top-ranking quantitative results in a public challenge, i.e., a median Dice similarity coefficient of 0.813 for the vessel wall and median Hausdorff distances of 0.552 mm and 0.776 mm for lumen and outer wall, respectively. Moreover, we show how the method improves over a conventional semantic segmentation approach. These results show that it is feasible to automatically obtain anatomically plausible segmentations of the carotid vessel wall with high accuracy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge