Kak Khee Yeung
Wall Shear Stress Estimation in Abdominal Aortic Aneurysms: Towards Generalisable Neural Surrogate Models
Jul 30, 2025Abstract:Abdominal aortic aneurysms (AAAs) are pathologic dilatations of the abdominal aorta posing a high fatality risk upon rupture. Studying AAA progression and rupture risk often involves in-silico blood flow modelling with computational fluid dynamics (CFD) and extraction of hemodynamic factors like time-averaged wall shear stress (TAWSS) or oscillatory shear index (OSI). However, CFD simulations are known to be computationally demanding. Hence, in recent years, geometric deep learning methods, operating directly on 3D shapes, have been proposed as compelling surrogates, estimating hemodynamic parameters in just a few seconds. In this work, we propose a geometric deep learning approach to estimating hemodynamics in AAA patients, and study its generalisability to common factors of real-world variation. We propose an E(3)-equivariant deep learning model utilising novel robust geometrical descriptors and projective geometric algebra. Our model is trained to estimate transient WSS using a dataset of CT scans of 100 AAA patients, from which lumen geometries are extracted and reference CFD simulations with varying boundary conditions are obtained. Results show that the model generalizes well within the distribution, as well as to the external test set. Moreover, the model can accurately estimate hemodynamics across geometry remodelling and changes in boundary conditions. Furthermore, we find that a trained model can be applied to different artery tree topologies, where new and unseen branches are added during inference. Finally, we find that the model is to a large extent agnostic to mesh resolution. These results show the accuracy and generalisation of the proposed model, and highlight its potential to contribute to hemodynamic parameter estimation in clinical practice.
Geometric deep learning for local growth prediction on abdominal aortic aneurysm surfaces
Jun 11, 2025Abstract:Abdominal aortic aneurysms (AAAs) are progressive focal dilatations of the abdominal aorta. AAAs may rupture, with a survival rate of only 20\%. Current clinical guidelines recommend elective surgical repair when the maximum AAA diameter exceeds 55 mm in men or 50 mm in women. Patients that do not meet these criteria are periodically monitored, with surveillance intervals based on the maximum AAA diameter. However, this diameter does not take into account the complex relation between the 3D AAA shape and its growth, making standardized intervals potentially unfit. Personalized AAA growth predictions could improve monitoring strategies. We propose to use an SE(3)-symmetric transformer model to predict AAA growth directly on the vascular model surface enriched with local, multi-physical features. In contrast to other works which have parameterized the AAA shape, this representation preserves the vascular surface's anatomical structure and geometric fidelity. We train our model using a longitudinal dataset of 113 computed tomography angiography (CTA) scans of 24 AAA patients at irregularly sampled intervals. After training, our model predicts AAA growth to the next scan moment with a median diameter error of 1.18 mm. We further demonstrate our model's utility to identify whether a patient will become eligible for elective repair within two years (acc = 0.93). Finally, we evaluate our model's generalization on an external validation set consisting of 25 CTAs from 7 AAA patients from a different hospital. Our results show that local directional AAA growth prediction from the vascular surface is feasible and may contribute to personalized surveillance strategies.
Active Learning for Deep Learning-Based Hemodynamic Parameter Estimation
Mar 05, 2025Abstract:Hemodynamic parameters such as pressure and wall shear stress play an important role in diagnosis, prognosis, and treatment planning in cardiovascular diseases. These parameters can be accurately computed using computational fluid dynamics (CFD), but CFD is computationally intensive. Hence, deep learning methods have been adopted as a surrogate to rapidly estimate CFD outcomes. A drawback of such data-driven models is the need for time-consuming reference CFD simulations for training. In this work, we introduce an active learning framework to reduce the number of CFD simulations required for the training of surrogate models, lowering the barriers to their deployment in new applications. We propose three distinct querying strategies to determine for which unlabeled samples CFD simulations should be obtained. These querying strategies are based on geometrical variance, ensemble uncertainty, and adherence to the physics governing fluid dynamics. We benchmark these methods on velocity field estimation in synthetic coronary artery bifurcations and find that they allow for substantial reductions in annotation cost. Notably, we find that our strategies reduce the number of samples required by up to 50% and make the trained models more robust to difficult cases. Our results show that active learning is a feasible strategy to increase the potential of deep learning-based CFD surrogates.
Global Control for Local SO-Equivariant Scale-Invariant Vessel Segmentation
Mar 22, 2024



Abstract:Personalized 3D vascular models can aid in a range of diagnostic, prognostic, and treatment-planning tasks relevant to cardiovascular disease management. Deep learning provides a means to automatically obtain such models. Ideally, a user should have control over the exact region of interest (ROI) to be included in a vascular model, and the model should be watertight and highly accurate. To this end, we propose a combination of a global controller leveraging voxel mask segmentations to provide boundary conditions for vessels of interest to a local, iterative vessel segmentation model. We introduce the preservation of scale- and rotational symmetries in the local segmentation model, leading to generalisation to vessels of unseen sizes and orientations. Combined with the global controller, this enables flexible 3D vascular model building, without additional retraining. We demonstrate the potential of our method on a dataset containing abdominal aortic aneurysms (AAAs). Our method performs on par with a state-of-the-art segmentation model in the segmentation of AAAs, iliac arteries and renal arteries, while providing a watertight, smooth surface segmentation. Moreover, we demonstrate that by adapting the global controller, we can easily extend vessel sections in the 3D model.
SIRE: scale-invariant, rotation-equivariant estimation of artery orientations using graph neural networks
Nov 09, 2023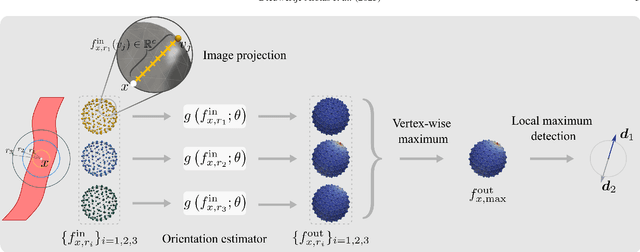
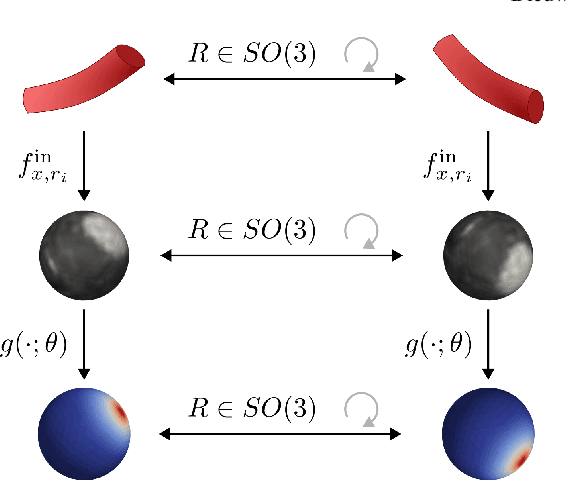
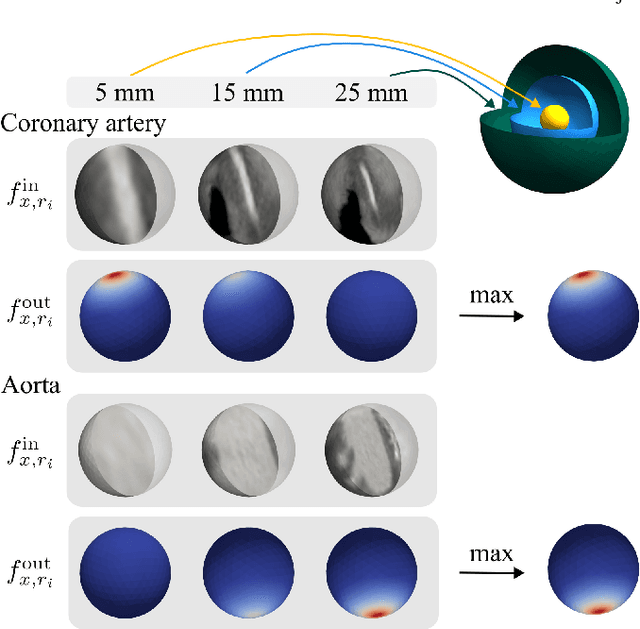
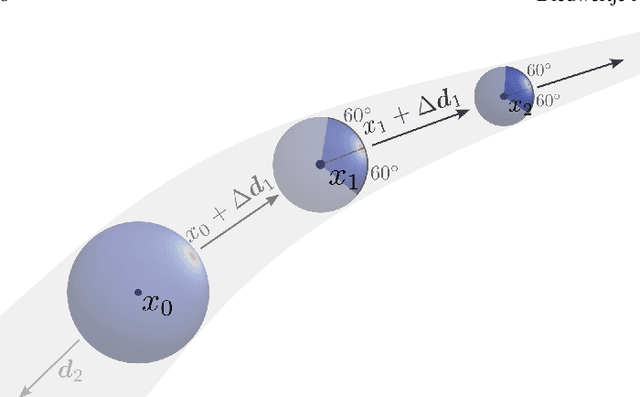
Abstract:Blood vessel orientation as visualized in 3D medical images is an important descriptor of its geometry that can be used for centerline extraction and subsequent segmentation and visualization. Arteries appear at many scales and levels of tortuosity, and determining their exact orientation is challenging. Recent works have used 3D convolutional neural networks (CNNs) for this purpose, but CNNs are sensitive to varying vessel sizes and orientations. We present SIRE: a scale-invariant, rotation-equivariant estimator for local vessel orientation. SIRE is modular and can generalise due to symmetry preservation. SIRE consists of a gauge equivariant mesh CNN (GEM-CNN) operating on multiple nested spherical meshes with different sizes in parallel. The features on each mesh are a projection of image intensities within the corresponding sphere. These features are intrinsic to the sphere and, in combination with the GEM-CNN, lead to SO(3)-equivariance. Approximate scale invariance is achieved by weight sharing and use of a symmetric maximum function to combine multi-scale predictions. Hence, SIRE can be trained with arbitrarily oriented vessels with varying radii to generalise to vessels with a wide range of calibres and tortuosity. We demonstrate the efficacy of SIRE using three datasets containing vessels of varying scales: the vascular model repository (VMR), the ASOCA coronary artery set, and a set of abdominal aortic aneurysms (AAAs). We embed SIRE in a centerline tracker which accurately tracks AAAs, regardless of the data SIRE is trained with. Moreover, SIRE can be used to track coronary arteries, even when trained only with AAAs. In conclusion, by incorporating SO(3) and scale symmetries, SIRE can determine the orientations of vessels outside of the training domain, forming a robust and data-efficient solution to geometric analysis of blood vessels in 3D medical images.
Implicit Neural Representations for Modeling of Abdominal Aortic Aneurysm Progression
Mar 02, 2023



Abstract:Abdominal aortic aneurysms (AAAs) are progressive dilatations of the abdominal aorta that, if left untreated, can rupture with lethal consequences. Imaging-based patient monitoring is required to select patients eligible for surgical repair. In this work, we present a model based on implicit neural representations (INRs) to model AAA progression. We represent the AAA wall over time as the zero-level set of a signed distance function (SDF), estimated by a multilayer perception that operates on space and time. We optimize this INR using automatically extracted segmentation masks in longitudinal CT data. This network is conditioned on spatiotemporal coordinates and represents the AAA surface at any desired resolution at any moment in time. Using regularization on spatial and temporal gradients of the SDF, we ensure proper interpolation of the AAA shape. We demonstrate the network's ability to produce AAA interpolations with average surface distances ranging between 0.72 and 2.52 mm from images acquired at highly irregular intervals. The results indicate that our model can accurately interpolate AAA shapes over time, with potential clinical value for a more personalised assessment of AAA progression.
Going Off-Grid: Continuous Implicit Neural Representations for 3D Vascular Modeling
Jul 29, 2022



Abstract:Personalised 3D vascular models are valuable for diagnosis, prognosis and treatment planning in patients with cardiovascular disease. Traditionally, such models have been constructed with explicit representations such as meshes and voxel masks, or implicit representations such as radial basis functions or atomic (tubular) shapes. Here, we propose to represent surfaces by the zero level set of their signed distance function (SDF) in a differentiable implicit neural representation (INR). This allows us to model complex vascular structures with a representation that is implicit, continuous, light-weight, and easy to integrate with deep learning algorithms. We here demonstrate the potential of this approach with three practical examples. First, we obtain an accurate and watertight surface for an abdominal aortic aneurysm (AAA) from CT images and show robust fitting from as little as 200 points on the surface. Second, we simultaneously fit nested vessel walls in a single INR without intersections. Third, we show how 3D models of individual arteries can be smoothly blended into a single watertight surface. Our results show that INRs are a flexible representation with potential for minimally interactive annotation and manipulation of complex vascular structures.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge