David Kersting
Mirror U-Net: Marrying Multimodal Fission with Multi-task Learning for Semantic Segmentation in Medical Imaging
Mar 13, 2023



Abstract:Positron Emission Tomography (PET) and Computer Tomography (CT) are routinely used together to detect tumors. PET/CT segmentation models can automate tumor delineation, however, current multimodal models do not fully exploit the complementary information in each modality, as they either concatenate PET and CT data or fuse them at the decision level. To combat this, we propose Mirror U-Net, which replaces traditional fusion methods with multimodal fission by factorizing the multimodal representation into modality-specific branches and an auxiliary multimodal decoder. At these branches, Mirror U-Net assigns a task tailored to each modality to reinforce unimodal features while preserving multimodal features in the shared representation. In contrast to previous methods that use either fission or multi-task learning, Mirror U-Net combines both paradigms in a unified framework. We explore various task combinations and examine which parameters to share in the model. We evaluate Mirror U-Net on the AutoPET PET/CT and on the multimodal MSD BrainTumor datasets, demonstrating its effectiveness in multimodal segmentation and achieving state-of-the-art performance on both datasets. Our code will be made publicly available.
AutoPET Challenge: Combining nn-Unet with Swin UNETR Augmented by Maximum Intensity Projection Classifier
Sep 02, 2022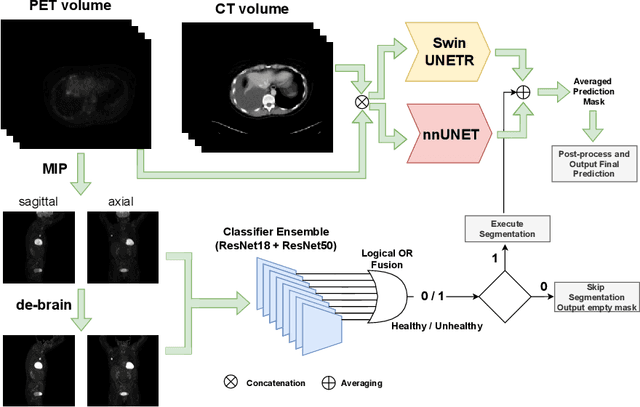
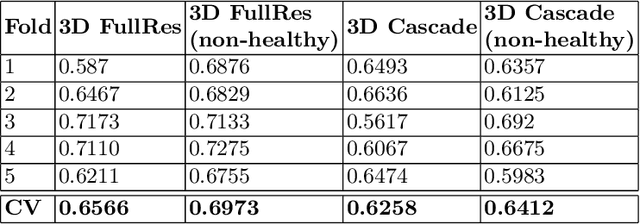
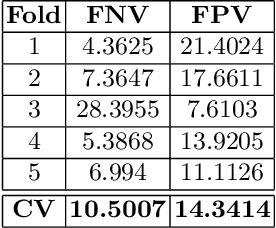
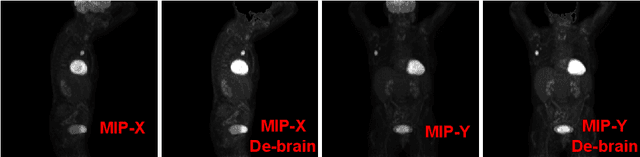
Abstract:Tumor volume and changes in tumor characteristics over time are important biomarkers for cancer therapy. In this context, FDG-PET/CT scans are routinely used for staging and re-staging of cancer, as the radiolabeled fluorodeoxyglucose is taken up in regions of high metabolism. Unfortunately, these regions with high metabolism are not specific to tumors and can also represent physiological uptake by normal functioning organs, inflammation, or infection, making detailed and reliable tumor segmentation in these scans a demanding task. This gap in research is addressed by the AutoPET challenge, which provides a public data set with FDG-PET/CT scans from 900 patients to encourage further improvement in this field. Our contribution to this challenge is an ensemble of two state-of-the-art segmentation models, the nn-Unet and the Swin UNETR, augmented by a maximum intensity projection classifier that acts like a gating mechanism. If it predicts the existence of lesions, both segmentations are combined by a late fusion approach. Our solution achieves a Dice score of 72.12\% on patients diagnosed with lung cancer, melanoma, and lymphoma in our cross-validation. Code: https://github.com/heiligerl/autopet_submission
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge