Daniel Coelho de Castro
Analysing the effectiveness of a generative model for semi-supervised medical image segmentation
Nov 03, 2022



Abstract:Image segmentation is important in medical imaging, providing valuable, quantitative information for clinical decision-making in diagnosis, therapy, and intervention. The state-of-the-art in automated segmentation remains supervised learning, employing discriminative models such as U-Net. However, training these models requires access to large amounts of manually labelled data which is often difficult to obtain in real medical applications. In such settings, semi-supervised learning (SSL) attempts to leverage the abundance of unlabelled data to obtain more robust and reliable models. Recently, generative models have been proposed for semantic segmentation, as they make an attractive choice for SSL. Their ability to capture the joint distribution over input images and output label maps provides a natural way to incorporate information from unlabelled images. This paper analyses whether deep generative models such as the SemanticGAN are truly viable alternatives to tackle challenging medical image segmentation problems. To that end, we thoroughly evaluate the segmentation performance, robustness, and potential subgroup disparities of discriminative and generative segmentation methods when applied to large-scale, publicly available chest X-ray datasets.
Stochastic Segmentation Networks: Modelling Spatially Correlated Aleatoric Uncertainty
Jun 10, 2020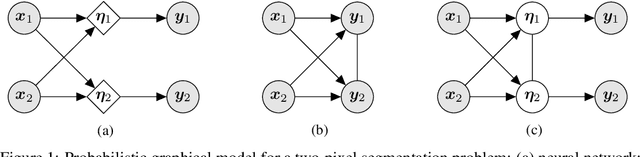


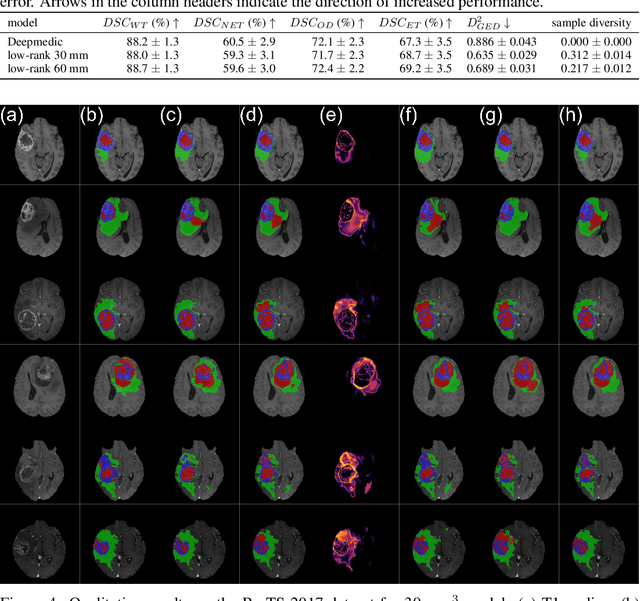
Abstract:In image segmentation, there is often more than one plausible solution for a given input. In medical imaging, for example, experts will often disagree about the exact location of object boundaries. Estimating this inherent uncertainty and predicting multiple plausible hypotheses is of great interest in many applications, yet this ability is lacking in most current deep learning methods. In this paper, we introduce stochastic segmentation networks (SSNs), an efficient probabilistic method for modelling aleatoric uncertainty with any image segmentation network architecture. In contrast to approaches that produce pixel-wise estimates, SSNs model joint distributions over entire label maps and thus can generate multiple spatially coherent hypotheses for a single image. By using a low-rank multivariate normal distribution over the logit space to model the probability of the label map given the image, we obtain a spatially consistent probability distribution that can be efficiently computed by a neural network without any changes to the underlying architecture. We tested our method on the segmentation of real-world medical data, including lung nodules in 2D CT and brain tumours in 3D multimodal MRI scans. SSNs outperform state-of-the-art for modelling correlated uncertainty in ambiguous images while being much simpler, more flexible, and more efficient.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge