Margherita Rosnati
Robust semi-supervised segmentation with timestep ensembling diffusion models
Nov 13, 2023



Abstract:Medical image segmentation is a challenging task, made more difficult by many datasets' limited size and annotations. Denoising diffusion probabilistic models (DDPM) have recently shown promise in modelling the distribution of natural images and were successfully applied to various medical imaging tasks. This work focuses on semi-supervised image segmentation using diffusion models, particularly addressing domain generalisation. Firstly, we demonstrate that smaller diffusion steps generate latent representations that are more robust for downstream tasks than larger steps. Secondly, we use this insight to propose an improved esembling scheme that leverages information-dense small steps and the regularising effect of larger steps to generate predictions. Our model shows significantly better performance in domain-shifted settings while retaining competitive performance in-domain. Overall, this work highlights the potential of DDPMs for semi-supervised medical image segmentation and provides insights into optimising their performance under domain shift.
Analysing the effectiveness of a generative model for semi-supervised medical image segmentation
Nov 03, 2022



Abstract:Image segmentation is important in medical imaging, providing valuable, quantitative information for clinical decision-making in diagnosis, therapy, and intervention. The state-of-the-art in automated segmentation remains supervised learning, employing discriminative models such as U-Net. However, training these models requires access to large amounts of manually labelled data which is often difficult to obtain in real medical applications. In such settings, semi-supervised learning (SSL) attempts to leverage the abundance of unlabelled data to obtain more robust and reliable models. Recently, generative models have been proposed for semantic segmentation, as they make an attractive choice for SSL. Their ability to capture the joint distribution over input images and output label maps provides a natural way to incorporate information from unlabelled images. This paper analyses whether deep generative models such as the SemanticGAN are truly viable alternatives to tackle challenging medical image segmentation problems. To that end, we thoroughly evaluate the segmentation performance, robustness, and potential subgroup disparities of discriminative and generative segmentation methods when applied to large-scale, publicly available chest X-ray datasets.
Automatic lesion analysis for increased efficiency in outcome prediction of traumatic brain injury
Aug 08, 2022



Abstract:The accurate prognosis for traumatic brain injury (TBI) patients is difficult yet essential to inform therapy, patient management, and long-term after-care. Patient characteristics such as age, motor and pupil responsiveness, hypoxia and hypotension, and radiological findings on computed tomography (CT), have been identified as important variables for TBI outcome prediction. CT is the acute imaging modality of choice in clinical practice because of its acquisition speed and widespread availability. However, this modality is mainly used for qualitative and semi-quantitative assessment, such as the Marshall scoring system, which is prone to subjectivity and human errors. This work explores the predictive power of imaging biomarkers extracted from routinely-acquired hospital admission CT scans using a state-of-the-art, deep learning TBI lesion segmentation method. We use lesion volumes and corresponding lesion statistics as inputs for an extended TBI outcome prediction model. We compare the predictive power of our proposed features to the Marshall score, independently and when paired with classic TBI biomarkers. We find that automatically extracted quantitative CT features perform similarly or better than the Marshall score in predicting unfavourable TBI outcomes. Leveraging automatic atlas alignment, we also identify frontal extra-axial lesions as important indicators of poor outcome. Our work may contribute to a better understanding of TBI, and provides new insights into how automated neuroimaging analysis can be used to improve prognostication after TBI.
MGP-AttTCN: An Interpretable Machine Learning Model for the Prediction of Sepsis
Sep 27, 2019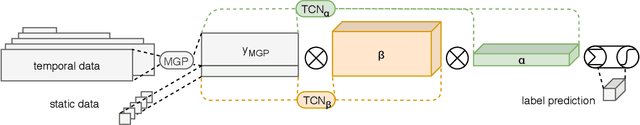
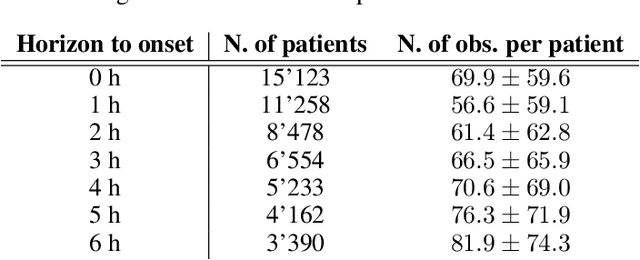
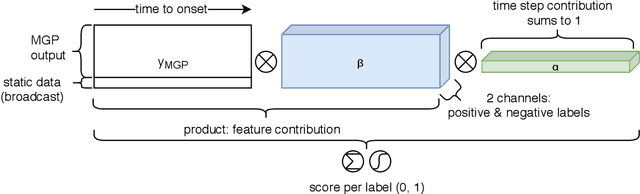
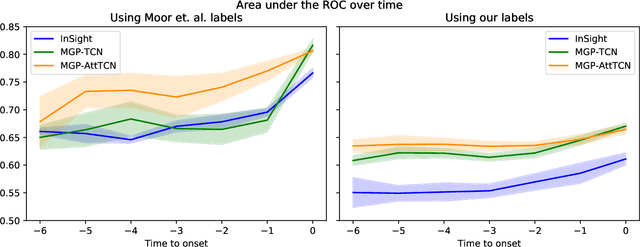
Abstract:With a mortality rate of 5.4 million lives worldwide every year and a healthcare cost of more than 16 billion dollars in the USA alone, sepsis is one of the leading causes of hospital mortality and an increasing concern in the ageing western world. Recently, medical and technological advances have helped re-define the illness criteria of this disease, which is otherwise poorly understood by the medical society. Together with the rise of widely accessible Electronic Health Records, the advances in data mining and complex nonlinear algorithms are a promising avenue for the early detection of sepsis. This work contributes to the research effort in the field of automated sepsis detection with an open-access labelling of the medical MIMIC-III data set. Moreover, we propose MGP-AttTCN: a joint multitask Gaussian Process and attention-based deep learning model to early predict the occurrence of sepsis in an interpretable manner. We show that our model outperforms the current state-of-the-art and present evidence that different labelling heuristics lead to discrepancies in task difficulty.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge