Corey McMillan
Surface-based parcellation and vertex-wise analysis of ultra high-resolution ex vivo 7 tesla MRI in neurodegenerative diseases
Mar 28, 2024



Abstract:Magnetic resonance imaging (MRI) is the standard modality to understand human brain structure and function in vivo (antemortem). Decades of research in human neuroimaging has led to the widespread development of methods and tools to provide automated volume-based segmentations and surface-based parcellations which help localize brain functions to specialized anatomical regions. Recently ex vivo (postmortem) imaging of the brain has opened-up avenues to study brain structure at sub-millimeter ultra high-resolution revealing details not possible to observe with in vivo MRI. Unfortunately, there has been limited methodological development in ex vivo MRI primarily due to lack of datasets and limited centers with such imaging resources. Therefore, in this work, we present one-of-its-kind dataset of 82 ex vivo T2w whole brain hemispheres MRI at 0.3 mm isotropic resolution spanning Alzheimer's disease and related dementias. We adapted and developed a fast and easy-to-use automated surface-based pipeline to parcellate, for the first time, ultra high-resolution ex vivo brain tissue at the native subject space resolution using the Desikan-Killiany-Tourville (DKT) brain atlas. This allows us to perform vertex-wise analysis in the template space and thereby link morphometry measures with pathology measurements derived from histology. We will open-source our dataset docker container, Jupyter notebooks for ready-to-use out-of-the-box set of tools and command line options to advance ex vivo MRI clinical brain imaging research on the project webpage.
Video and Synthetic MRI Pre-training of 3D Vision Architectures for Neuroimage Analysis
Sep 09, 2023Abstract:Transfer learning represents a recent paradigm shift in the way we build artificial intelligence (AI) systems. In contrast to training task-specific models, transfer learning involves pre-training deep learning models on a large corpus of data and minimally fine-tuning them for adaptation to specific tasks. Even so, for 3D medical imaging tasks, we do not know if it is best to pre-train models on natural images, medical images, or even synthetically generated MRI scans or video data. To evaluate these alternatives, here we benchmarked vision transformers (ViTs) and convolutional neural networks (CNNs), initialized with varied upstream pre-training approaches. These methods were then adapted to three unique downstream neuroimaging tasks with a range of difficulty: Alzheimer's disease (AD) and Parkinson's disease (PD) classification, "brain age" prediction. Experimental tests led to the following key observations: 1. Pre-training improved performance across all tasks including a boost of 7.4% for AD classification and 4.6% for PD classification for the ViT and 19.1% for PD classification and reduction in brain age prediction error by 1.26 years for CNNs, 2. Pre-training on large-scale video or synthetic MRI data boosted performance of ViTs, 3. CNNs were robust in limited-data settings, and in-domain pretraining enhanced their performances, 4. Pre-training improved generalization to out-of-distribution datasets and sites. Overall, we benchmarked different vision architectures, revealing the value of pre-training them with emerging datasets for model initialization. The resulting pre-trained models can be adapted to a range of downstream neuroimaging tasks, even when training data for the target task is limited.
Automated deep learning segmentation of high-resolution 7 T ex vivo MRI for quantitative analysis of structure-pathology correlations in neurodegenerative diseases
Mar 21, 2023



Abstract:Ex vivo MRI of the brain provides remarkable advantages over in vivo MRI for visualizing and characterizing detailed neuroanatomy, and helps to link microscale histology studies with morphometric measurements. However, automated segmentation methods for brain mapping in ex vivo MRI are not well developed, primarily due to limited availability of labeled datasets, and heterogeneity in scanner hardware and acquisition protocols. In this work, we present a high resolution dataset of 37 ex vivo post-mortem human brain tissue specimens scanned on a 7T whole-body MRI scanner. We developed a deep learning pipeline to segment the cortical mantle by benchmarking the performance of nine deep neural architectures. We then segment the four subcortical structures: caudate, putamen, globus pallidus, and thalamus; white matter hyperintensities, and the normal appearing white matter. We show excellent generalizing capabilities across whole brain hemispheres in different specimens, and also on unseen images acquired at different magnetic field strengths and different imaging sequence. We then compute volumetric and localized cortical thickness measurements across key regions, and link them with semi-quantitative neuropathological ratings. Our code, containerized executables, and the processed datasets are publicly available at: https://github.com/Pulkit-Khandelwal/upenn-picsl-brain-ex-vivo.
Curriculum Based Multi-Task Learning for Parkinson's Disease Detection
Feb 27, 2023Abstract:There is great interest in developing radiological classifiers for diagnosis, staging, and predictive modeling in progressive diseases such as Parkinson's disease (PD), a neurodegenerative disease that is difficult to detect in its early stages. Here we leverage severity-based meta-data on the stages of disease to define a curriculum for training a deep convolutional neural network (CNN). Typically, deep learning networks are trained by randomly selecting samples in each mini-batch. By contrast, curriculum learning is a training strategy that aims to boost classifier performance by starting with examples that are easier to classify. Here we define a curriculum to progressively increase the difficulty of the training data corresponding to the Hoehn and Yahr (H&Y) staging system for PD (total N=1,012; 653 PD patients, 359 controls; age range: 20.0-84.9 years). Even with our multi-task setting using pre-trained CNNs and transfer learning, PD classification based on T1-weighted (T1-w) MRI was challenging (ROC AUC: 0.59-0.65), but curriculum training boosted performance (by 3.9%) compared to our baseline model. Future work with multimodal imaging may further boost performance.
Predicting Brain Age using Transferable coVariance Neural Networks
Oct 28, 2022

Abstract:The deviation between chronological age and biological age is a well-recognized biomarker associated with cognitive decline and neurodegeneration. Age-related and pathology-driven changes to brain structure are captured by various neuroimaging modalities. These datasets are characterized by high dimensionality as well as collinearity, hence applications of graph neural networks in neuroimaging research routinely use sample covariance matrices as graphs. We have recently studied covariance neural networks (VNNs) that operate on sample covariance matrices using the architecture derived from graph convolutional networks, and we showed VNNs enjoy significant advantages over traditional data analysis approaches. In this paper, we demonstrate the utility of VNNs in inferring brain age using cortical thickness data. Furthermore, our results show that VNNs exhibit multi-scale and multi-site transferability for inferring {brain age}. In the context of brain age in Alzheimer's disease (AD), our experiments show that i) VNN outputs are interpretable as brain age predicted using VNNs is significantly elevated for AD with respect to healthy subjects for different datasets; and ii) VNNs can be transferable, i.e., VNNs trained on one dataset can be transferred to another dataset with different dimensions without retraining for brain age prediction.
coVariance Neural Networks
May 31, 2022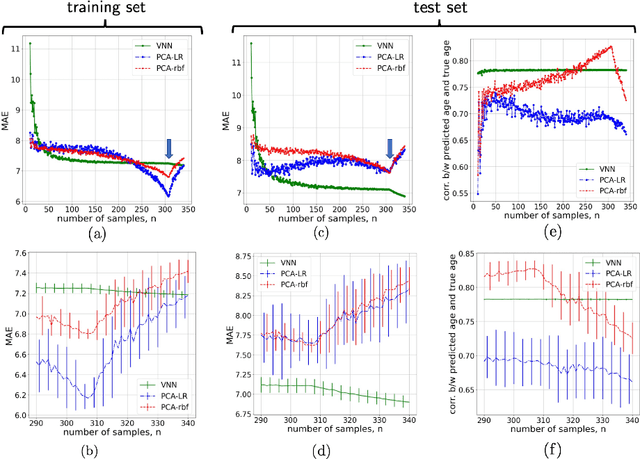
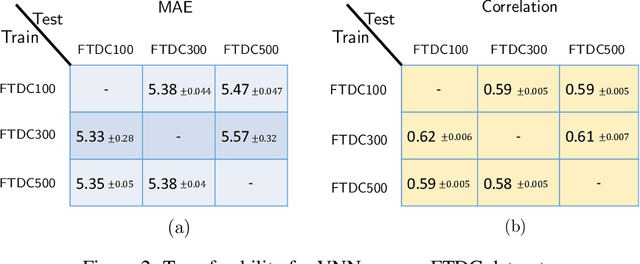
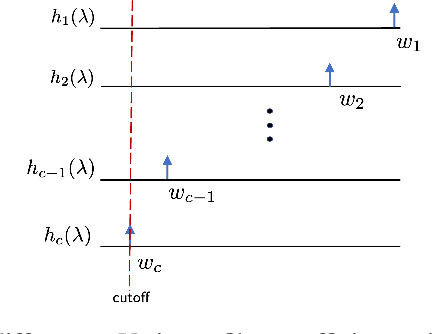
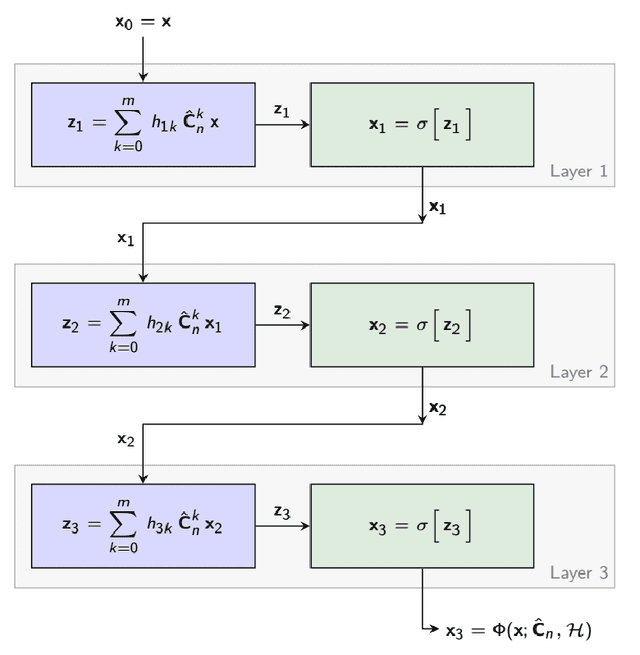
Abstract:Graph neural networks (GNN) are an effective framework that exploit inter-relationships within graph-structured data for learning. Principal component analysis (PCA) involves the projection of data on the eigenspace of the covariance matrix and draws similarities with the graph convolutional filters in GNNs. Motivated by this observation, we propose a GNN architecture, called coVariance neural network (VNN), that operates on sample covariance matrices as graphs. We theoretically establish the stability of VNNs to perturbations in the covariance matrix, thus, implying an advantage over standard PCA-based data analysis approaches that are prone to instability due to principal components associated with close eigenvalues. Our experiments on real-world datasets validate our theoretical results and show that VNN performance is indeed more stable than PCA-based statistical approaches. Moreover, our experiments on multi-resolution datasets also demonstrate that VNNs are amenable to transferability of performance over covariance matrices of different dimensions; a feature that is infeasible for PCA-based approaches.
Gray Matter Segmentation in Ultra High Resolution 7 Tesla ex vivo T2w MRI of Human Brain Hemispheres
Oct 14, 2021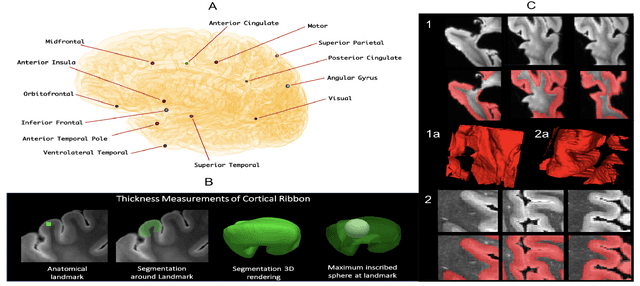
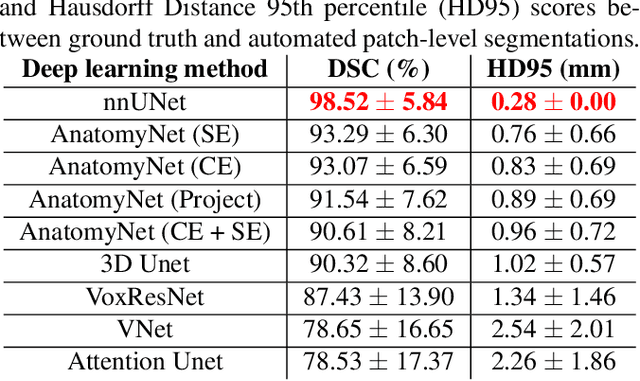
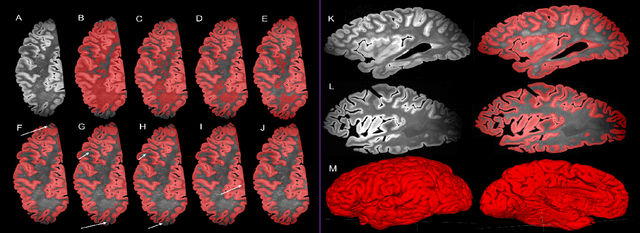
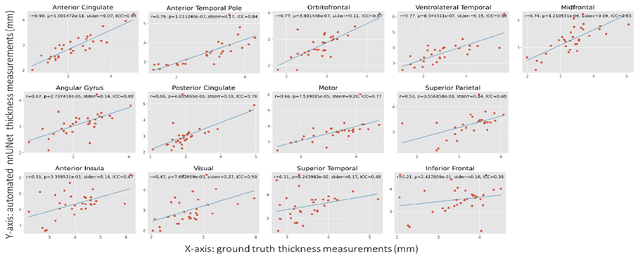
Abstract:Ex vivo MRI of the brain provides remarkable advantages over in vivo MRI for visualizing and characterizing detailed neuroanatomy. However, automated cortical segmentation methods in ex vivo MRI are not well developed, primarily due to limited availability of labeled datasets, and heterogeneity in scanner hardware and acquisition protocols. In this work, we present a high resolution 7 Tesla dataset of 32 ex vivo human brain specimens. We benchmark the cortical mantle segmentation performance of nine neural network architectures, trained and evaluated using manually-segmented 3D patches sampled from specific cortical regions, and show excellent generalizing capabilities across whole brain hemispheres in different specimens, and also on unseen images acquired at different magnetic field strength and imaging sequences. Finally, we provide cortical thickness measurements across key regions in 3D ex vivo human brain images. Our code and processed datasets are publicly available at https://github.com/Pulkit-Khandelwal/picsl-ex-vivo-segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge