Claire Gowdy
A cascaded deep network for automated tumor detection and segmentation in clinical PET imaging of diffuse large B-cell lymphoma
Mar 11, 2024Abstract:Accurate detection and segmentation of diffuse large B-cell lymphoma (DLBCL) from PET images has important implications for estimation of total metabolic tumor volume, radiomics analysis, surgical intervention and radiotherapy. Manual segmentation of tumors in whole-body PET images is time-consuming, labor-intensive and operator-dependent. In this work, we develop and validate a fast and efficient three-step cascaded deep learning model for automated detection and segmentation of DLBCL tumors from PET images. As compared to a single end-to-end network for segmentation of tumors in whole-body PET images, our three-step model is more effective (improves 3D Dice score from 58.9% to 78.1%) since each of its specialized modules, namely the slice classifier, the tumor detector and the tumor segmentor, can be trained independently to a high degree of skill to carry out a specific task, rather than a single network with suboptimal performance on overall segmentation.
* 8 pages, 3 figures, 3 tables
Comprehensive Evaluation and Insights into the Use of Deep Neural Networks to Detect and Quantify Lymphoma Lesions in PET/CT Images
Nov 16, 2023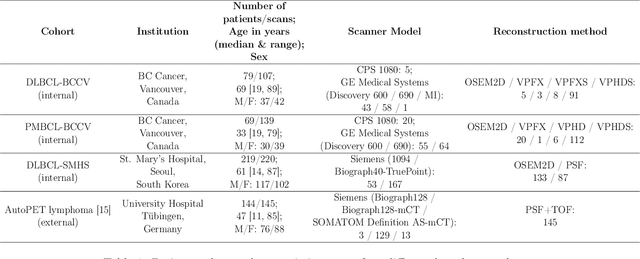
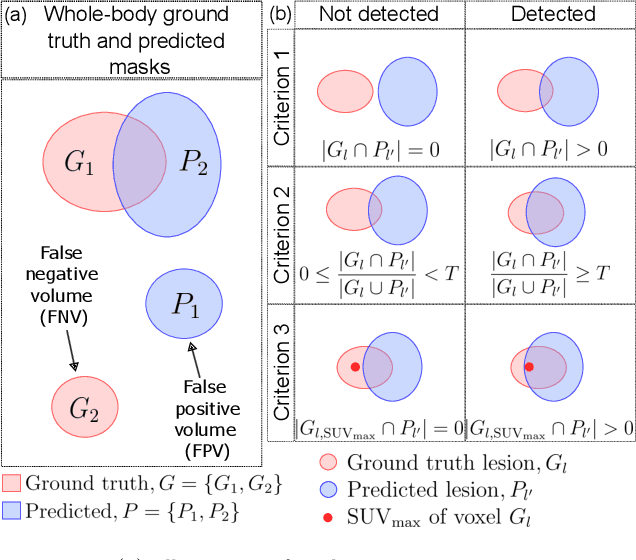
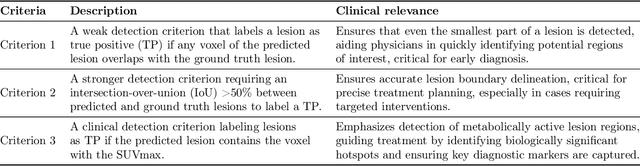
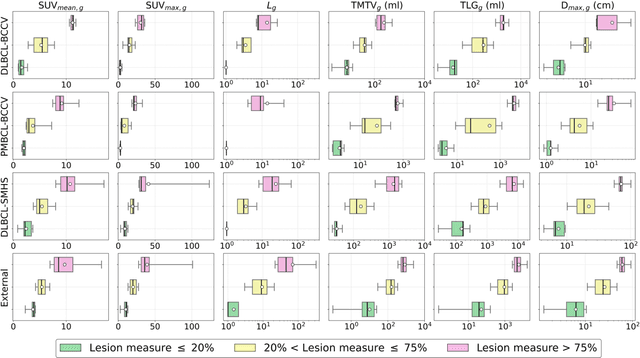
Abstract:This study performs comprehensive evaluation of four neural network architectures (UNet, SegResNet, DynUNet, and SwinUNETR) for lymphoma lesion segmentation from PET/CT images. These networks were trained, validated, and tested on a diverse, multi-institutional dataset of 611 cases. Internal testing (88 cases; total metabolic tumor volume (TMTV) range [0.52, 2300] ml) showed SegResNet as the top performer with a median Dice similarity coefficient (DSC) of 0.76 and median false positive volume (FPV) of 4.55 ml; all networks had a median false negative volume (FNV) of 0 ml. On the unseen external test set (145 cases with TMTV range: [0.10, 2480] ml), SegResNet achieved the best median DSC of 0.68 and FPV of 21.46 ml, while UNet had the best FNV of 0.41 ml. We assessed reproducibility of six lesion measures, calculated their prediction errors, and examined DSC performance in relation to these lesion measures, offering insights into segmentation accuracy and clinical relevance. Additionally, we introduced three lesion detection criteria, addressing the clinical need for identifying lesions, counting them, and segmenting based on metabolic characteristics. We also performed expert intra-observer variability analysis revealing the challenges in segmenting ``easy'' vs. ``hard'' cases, to assist in the development of more resilient segmentation algorithms. Finally, we performed inter-observer agreement assessment underscoring the importance of a standardized ground truth segmentation protocol involving multiple expert annotators. Code is available at: https://github.com/microsoft/lymphoma-segmentation-dnn
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge