Christina B. Lund
Biomedical image analysis competitions: The state of current participation practice
Dec 16, 2022Abstract:The number of international benchmarking competitions is steadily increasing in various fields of machine learning (ML) research and practice. So far, however, little is known about the common practice as well as bottlenecks faced by the community in tackling the research questions posed. To shed light on the status quo of algorithm development in the specific field of biomedical imaging analysis, we designed an international survey that was issued to all participants of challenges conducted in conjunction with the IEEE ISBI 2021 and MICCAI 2021 conferences (80 competitions in total). The survey covered participants' expertise and working environments, their chosen strategies, as well as algorithm characteristics. A median of 72% challenge participants took part in the survey. According to our results, knowledge exchange was the primary incentive (70%) for participation, while the reception of prize money played only a minor role (16%). While a median of 80 working hours was spent on method development, a large portion of participants stated that they did not have enough time for method development (32%). 25% perceived the infrastructure to be a bottleneck. Overall, 94% of all solutions were deep learning-based. Of these, 84% were based on standard architectures. 43% of the respondents reported that the data samples (e.g., images) were too large to be processed at once. This was most commonly addressed by patch-based training (69%), downsampling (37%), and solving 3D analysis tasks as a series of 2D tasks. K-fold cross-validation on the training set was performed by only 37% of the participants and only 50% of the participants performed ensembling based on multiple identical models (61%) or heterogeneous models (39%). 48% of the respondents applied postprocessing steps.
Leveraging Clinical Characteristics for Improved Deep Learning-Based Kidney Tumor Segmentation on CT
Sep 13, 2021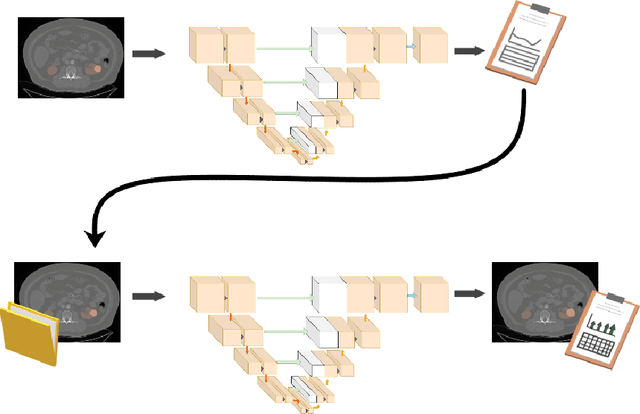

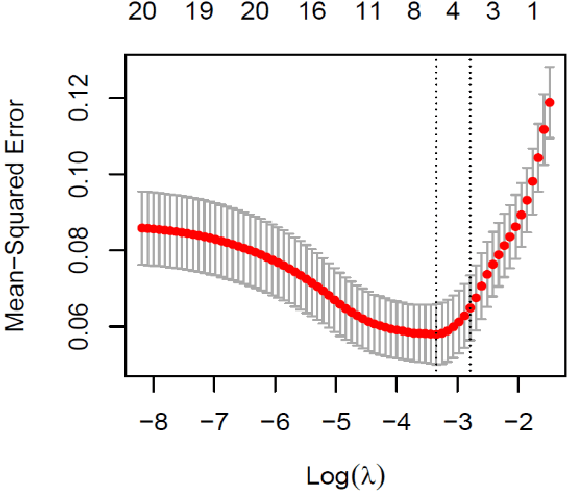

Abstract:This paper assesses whether using clinical characteristics in addition to imaging can improve automated segmentation of kidney cancer on contrast-enhanced computed tomography (CT). A total of 300 kidney cancer patients with contrast-enhanced CT scans and clinical characteristics were included. A baseline segmentation of the kidney cancer was performed using a 3D U-Net. Input to the U-Net were the contrast-enhanced CT images, output were segmentations of kidney, kidney tumors, and kidney cysts. A cognizant sampling strategy was used to leverage clinical characteristics for improved segmentation. To this end, a Least Absolute Shrinkage and Selection Operator (LASSO) was used. Segmentations were evaluated using Dice and Surface Dice. Improvement in segmentation was assessed using Wilcoxon signed rank test. The baseline 3D U-Net showed a segmentation performance of 0.90 for kidney and kidney masses, i.e., kidney, tumor, and cyst, 0.29 for kidney masses, and 0.28 for kidney tumor, while the 3D U-Net trained with cognizant sampling enhanced the segmentation performance and reached Dice scores of 0.90, 0.39, and 0.38 respectively. To conclude, the cognizant sampling strategy leveraging the clinical characteristics significantly improved kidney cancer segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge