Changhuei Yang
Digital Modeling of Spatial Pathway Activity from Histology Reveals Tumor Microenvironment Heterogeneity
Dec 18, 2025
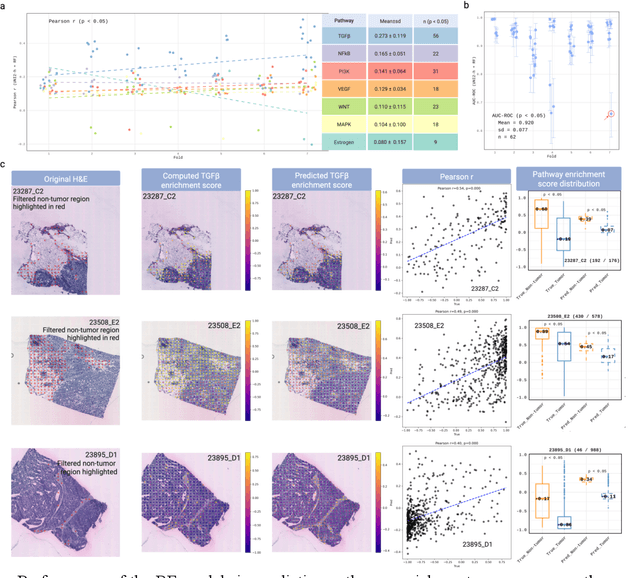
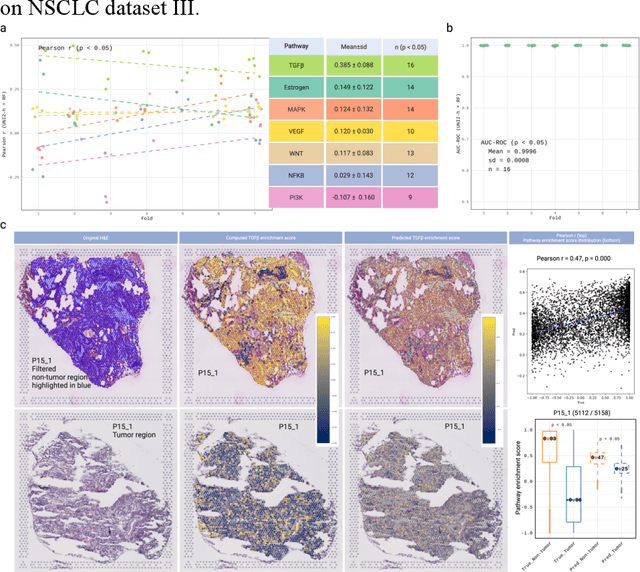
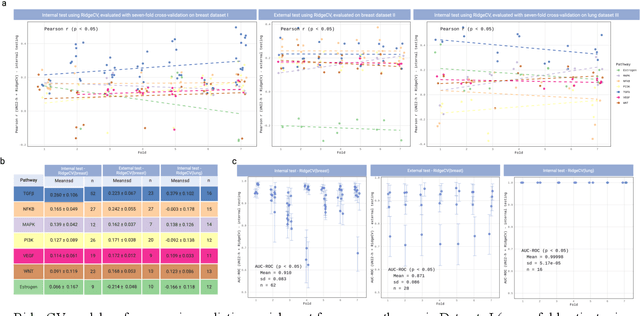
Abstract:Spatial transcriptomics (ST) enables simultaneous mapping of tissue morphology and spatially resolved gene expression, offering unique opportunities to study tumor microenvironment heterogeneity. Here, we introduce a computational framework that predicts spatial pathway activity directly from hematoxylin-and-eosin-stained histology images at microscale resolution 55 and 100 um. Using image features derived from a computational pathology foundation model, we found that TGFb signaling was the most accurately predicted pathway across three independent breast and lung cancer ST datasets. In 87-88% of reliably predicted cases, the resulting spatial TGFb activity maps reflected the expected contrast between tumor and adjacent non-tumor regions, consistent with the known role of TGFb in regulating interactions within the tumor microenvironment. Notably, linear and nonlinear predictive models performed similarly, suggesting that image features may relate to pathway activity in a predominantly linear fashion or that nonlinear structure is small relative to measurement noise. These findings demonstrate that features extracted from routine histopathology may recover spatially coherent and biologically interpretable pathway patterns, offering a scalable strategy for integrating image-based inference with ST information in tumor microenvironment studies.
Impact of Stain Variation and Color Normalization for Prognostic Predictions in Pathology
Sep 12, 2024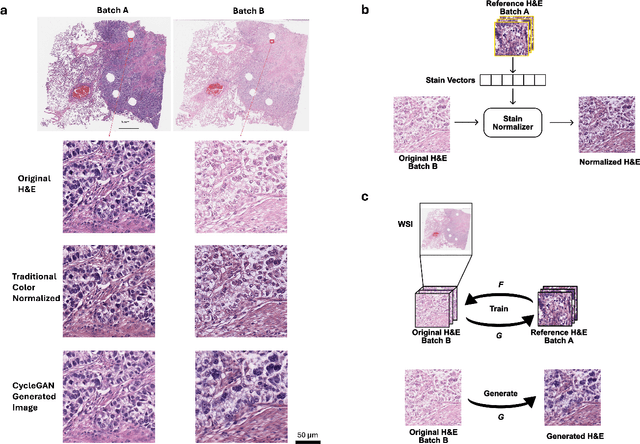
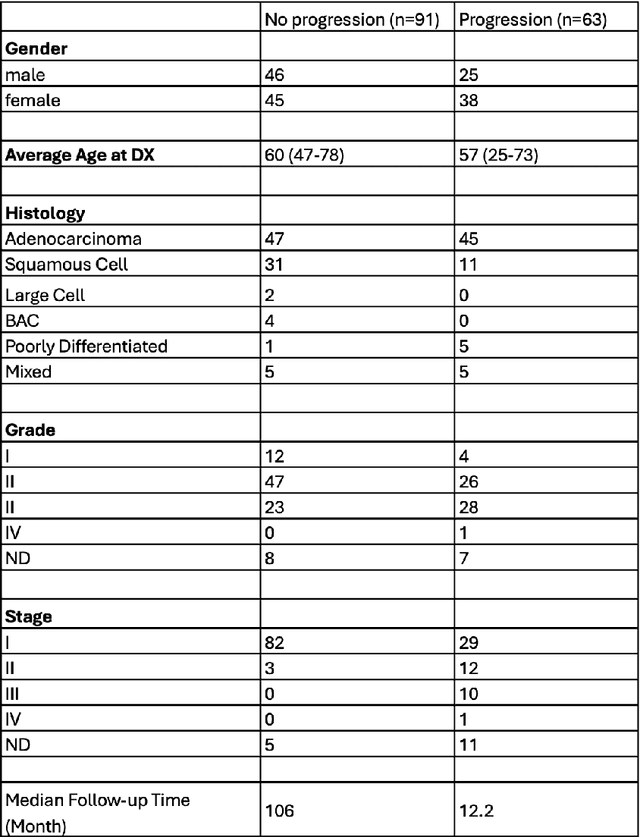
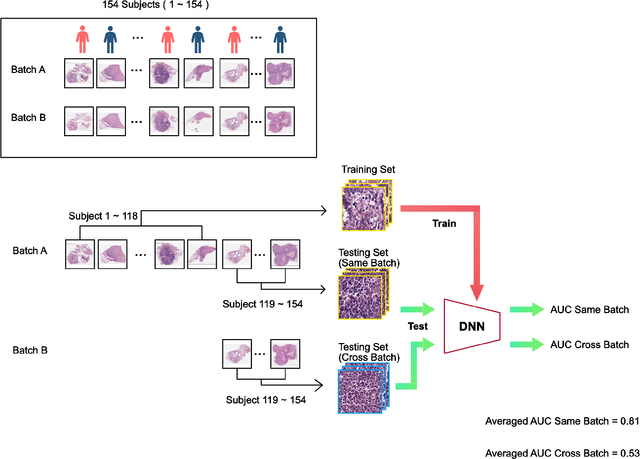
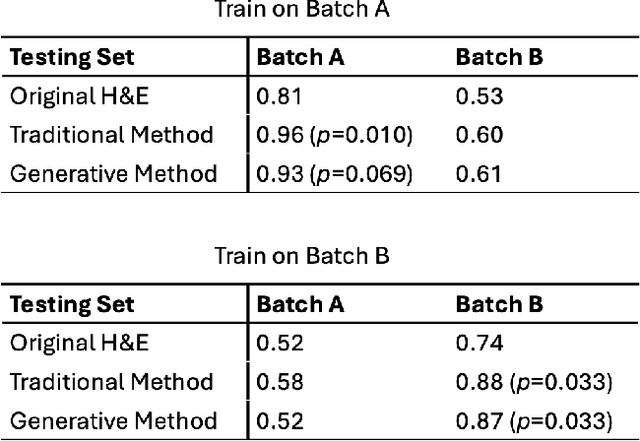
Abstract:In recent years, deep neural networks (DNNs) have demonstrated remarkable performance in pathology applications, potentially even outperforming expert pathologists due to their ability to learn subtle features from large datasets. One complication in preparing digital pathology datasets for DNN tasks is variation in tinctorial qualities. A common way to address this is to perform stain normalization on the images. In this study, we show that a well-trained DNN model trained on one batch of histological slides failed to generalize to another batch prepared at a different time from the same tissue blocks, even when stain normalization methods were applied. This study used sample data from a previously reported DNN that was able to identify patients with early stage non-small cell lung cancer (NSCLC) whose tumors did and did not metastasize, with high accuracy, based on training and then testing of digital images from H&E stained primary tumor tissue sections processed at the same time. In this study we obtained a new series of histologic slides from the adjacent recuts of same tissue blocks processed in the same lab but at a different time. We found that the DNN trained on the either batch of slides/images was unable to generalize and failed to predict progression in the other batch of slides/images (AUC_cross-batch = 0.52 - 0.53 compared to AUC_same-batch = 0.74 - 0.81). The failure to generalize did not improve even when the tinctorial difference correction were made through either traditional color-tuning or stain normalization with the help of a Cycle Generative Adversarial Network (CycleGAN) process. This highlights the need to develop an entirely new way to process and collect consistent microscopy images from histologic slides that can be used to both train and allow for the general application of predictive DNN algorithms.
Efficient, gigapixel-scale, aberration-free whole slide scanner using angular ptychographic imaging with closed-form solution
Jul 29, 2024



Abstract:Whole slide imaging provides a wide field-of-view (FOV) across cross-sections of biopsy or surgery samples, significantly facilitating pathological analysis and clinical diagnosis. Such high-quality images that enable detailed visualization of cellular and tissue structures are essential for effective patient care and treatment planning. To obtain such high-quality images for pathology applications, there is a need for scanners with high spatial bandwidth products, free from aberrations, and without the requirement for z-scanning. Here we report a whole slide imaging system based on angular ptychographic imaging with a closed-form solution (WSI-APIC), which offers efficient, tens-of-gigapixels, large-FOV, aberration-free imaging. WSI-APIC utilizes oblique incoherent illumination for initial high-level segmentation, thereby bypassing unnecessary scanning of the background regions and enhancing image acquisition efficiency. A GPU-accelerated APIC algorithm analytically reconstructs phase images with effective digital aberration corrections and improved optical resolutions. Moreover, an auto-stitching technique based on scale-invariant feature transform ensures the seamless concatenation of whole slide phase images. In our experiment, WSI-APIC achieved an optical resolution of 772 nm using a 10x/0.25 NA objective lens and captures 80-gigapixel aberration-free phase images for a standard 76.2 mm x 25.4 mm microscopic slide.
Correlating Stroke Risk with Non-Invasive Tracing of Brain Blood Dynamic via a Portable Speckle Contrast Optical Spectroscopy Laser Device
Jul 23, 2024Abstract:Stroke poses a significant global health threat, with millions affected annually, leading to substantial morbidity and mortality. Current stroke risk assessment for the general population relies on markers such as demographics, blood tests, and comorbidities. A minimally invasive, clinically scalable, and cost-effective way to directly measure cerebral blood flow presents an opportunity. This opportunity has potential to positively impact effective stroke risk assessment prevention and intervention. Physiological changes in the cerebral vascular system, particularly in response to carbon dioxide level changes and oxygen deprivation, such as during breath-holding, can offer insights into stroke risk assessment. However, existing methods for measuring cerebral perfusion reserve, such as blood flow and blood volume changes, are limited by either invasiveness or impracticality. Here, we propose a transcranial approach using speckle contrast optical spectroscopy (SCOS) to non-invasively monitor regional changes in brain blood flow and volume during breath-holding. Our study, conducted on 50 individuals classified into two groups (low-risk and higher-risk for stroke), shows significant differences in blood dynamic changes during breath-holding between the two groups, providing physiological insights for stroke risk assessment using a non-invasive quantification paradigm. Given its cost-effectiveness, scalability, portability, and simplicity, this laser-centric tool has significant potential in enhancing the pre-screening of stroke and mitigating strokes in the general population through early diagnosis and intervention.
Length-scale study in deep learning prediction for non-small cell lung cancer brain metastasis
Jun 01, 2024Abstract:Deep learning assisted digital pathology has the potential to impact clinical practice in significant ways. In recent studies, deep neural network (DNN) enabled analysis outperforms human pathologists. Increasing sizes and complexity of the DNN architecture generally improves performance at the cost of DNN's explainability. For pathology, this lack of DNN explainability is particularly problematic as it hinders the broader clinical interpretation of the pathology features that may provide physiological disease insights. To better assess the features that DNN uses in developing predictive algorithms to interpret digital microscopic images, we sought to understand the role of resolution and tissue scale and here describe a novel method for studying the predictive feature length-scale that underpins a DNN's predictive power. We applied the method to study a DNN's predictive capability in the case example of brain metastasis prediction from early-stage non-small-cell lung cancer biopsy slides. The study highlights the DNN attention in the brain metastasis prediction targeting both cellular scale (resolution) and tissue scale features on H&E-stained histological whole slide images. At the cellular scale, we see that DNN's predictive power is progressively increased at higher resolution (i.e., lower resolvable feature length) and is largely lost when the resolvable feature length is longer than 5 microns. In addition, DNN uses more macro-scale features (maximal feature length) associated with tissue organization/architecture and is optimized when assessing visual fields larger than 41 microns. This study for the first time demonstrates the length-scale requirements necessary for optimal DNN learning on digital whole slide images.
Single-shot volumetric fluorescence imaging with neural fields
May 16, 2024Abstract:Single-shot volumetric fluorescence (SVF) imaging offers a significant advantage over traditional imaging methods that require scanning across multiple axial planes as it can capture biological processes with high temporal resolution across a large field of view. Existing SVF imaging methods often require large, complex point spread functions (PSFs) to meet the multiplexing requirements of compressed sensing, which limits the signal-to-noise ratio, resolution and/or field of view. In this paper, we introduce the QuadraPol PSF combined with neural fields, a novel approach for SVF imaging. This method utilizes a cost-effective custom polarizer at the back focal plane and a polarization camera to detect fluorescence, effectively encoding the 3D scene within a compact PSF without depth ambiguity. Additionally, we propose a reconstruction algorithm based on the neural fields technique that addresses the inaccuracies of phase retrieval methods used to correct imaging system aberrations. This algorithm combines the accuracy of experimental PSFs with the long depth of field of computationally generated retrieved PSFs. QuadraPol PSF, combined with neural fields, significantly reduces the acquisition time of a conventional fluorescence microscope by approximately 20 times and captures a 100 mm$^3$ cubic volume in one shot. We validate the effectiveness of both our hardware and algorithm through all-in-focus imaging of bacterial colonies on sand surfaces and visualization of plant root morphology. Our approach offers a powerful tool for advancing biological research and ecological studies.
A compact and cost-effective laser-powered speckle visibility spectroscopy device for measuring cerebral blood flow
Jan 29, 2024



Abstract:In the realm of cerebrovascular monitoring, primary metrics typically include blood pressure, which influences cerebral blood flow (CBF) and is contingent upon vessel radius. Measuring CBF non-invasively poses a persistent challenge, primarily attributed to the difficulty of accessing and obtaining signal from the brain. This study aims to introduce a compact speckle visibility spectroscopy (SVS) device designed for non-invasive CBF measurements, offering cost-effectiveness and scalability while tracking CBF with remarkable sensitivity and temporal resolution. The wearable hardware has a modular design approach consisting solely of a laser diode as the source and a meticulously selected board camera as the detector. They both can be easily placed on the head of a subject to measure CBF with no additional optical elements. The SVS device can achieve a sampling rate of 80 Hz with minimal susceptibility to external disturbances. The device also achieves better SNR compared with traditional fiber-based SVS devices, capturing about 70 times more signal and showing superior stability and reproducibility. It is designed to be paired and distributed in multiple configurations around the head, and measure signals that exceed the quality of prior optical CBF measurement techniques. Given its cost-effectiveness, scalability, and simplicity, this laser-centric tool offers significant potential in advancing non-invasive cerebral monitoring technologies.
FPM-INR: Fourier ptychographic microscopy image stack reconstruction using implicit neural representations
Oct 31, 2023
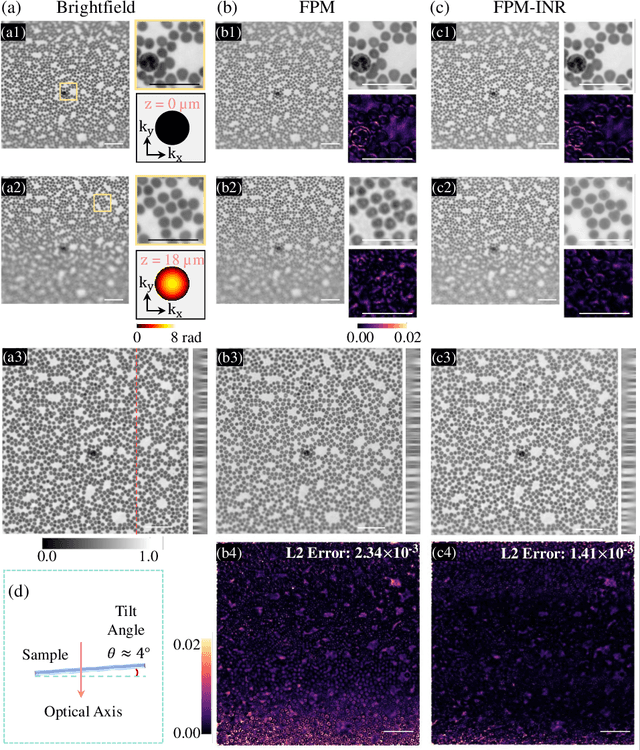
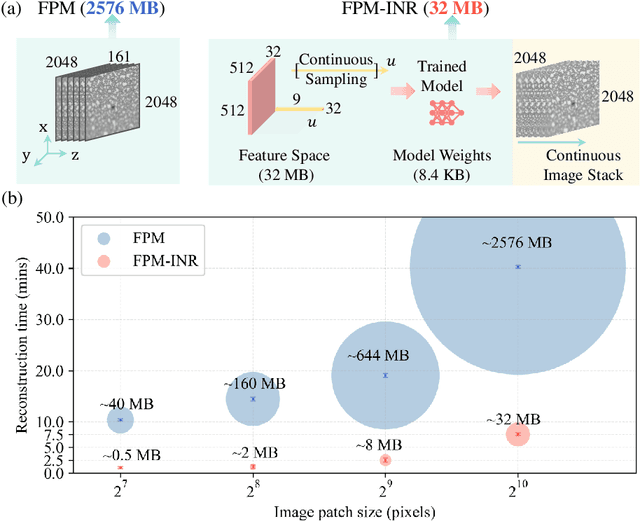
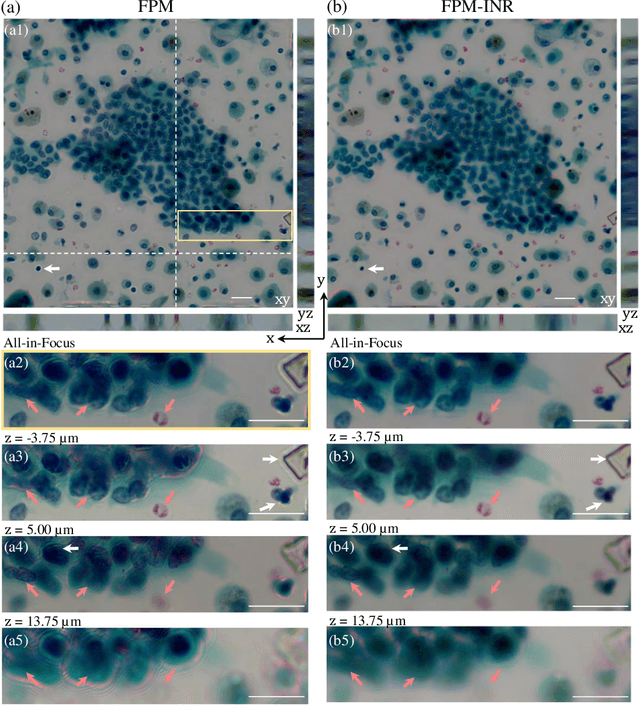
Abstract:Image stacks provide invaluable 3D information in various biological and pathological imaging applications. Fourier ptychographic microscopy (FPM) enables reconstructing high-resolution, wide field-of-view image stacks without z-stack scanning, thus significantly accelerating image acquisition. However, existing FPM methods take tens of minutes to reconstruct and gigabytes of memory to store a high-resolution volumetric scene, impeding fast gigapixel-scale remote digital pathology. While deep learning approaches have been explored to address this challenge, existing methods poorly generalize to novel datasets and can produce unreliable hallucinations. This work presents FPM-INR, a compact and efficient framework that integrates physics-based optical models with implicit neural representations (INR) to represent and reconstruct FPM image stacks. FPM-INR is agnostic to system design or sample types and does not require external training data. In our demonstrated experiments, FPM-INR substantially outperforms traditional FPM algorithms with up to a 25-fold increase in speed and an 80-fold reduction in memory usage for continuous image stack representations.
High-resolution, large field-of-view label-free imaging via aberration-corrected, closed-form complex field reconstruction
Sep 01, 2023Abstract:Computational imaging methods empower modern microscopy with the ability of producing high-resolution, large field-of-view, aberration-free images. One of the dominant computational label-free imaging methods, Fourier ptychographic microscopy (FPM), effectively increases the spatial-bandwidth product of conventional microscopy by using multiple tilted illuminations to achieve high-throughput imaging. However, its iterative reconstruction method is prone to parameter selection, can be computationally expensive and tends to fail under excessive aberrations. Recently, spatial Kramers-Kronig methods show it is possible to analytically reconstruct complex field but lacks the ability of correcting aberrations or providing extended resolution enhancement. Here, we present a closed-form method, termed APIC, which weds the strengths of both methods. A new analytical phase retrieval framework is established in APIC, which demonstrates, for the first time, the feasibility of analytically reconstructing the complex field associated with darkfield measurements. In addition, APIC can analytically retrieve complex aberrations of an imaging system with no additional hardware. By avoiding iterative algorithms, APIC requires no human designed convergence metric and always obtains a closed-form complex field solution. The faithfulness and correctness of APIC's reconstruction are guaranteed due to its analytical nature. We experimentally demonstrate that APIC gives correct reconstruction result while FPM fails to do so when constrained to the same number of measurements. Meanwhile, APIC achieves 2.8 times faster computation using image tile size of 256 (length-wise). We also demonstrate APIC is unprecedentedly robust against aberrations compared to FPM - APIC is capable of addressing aberration whose maximal phase difference exceeds 3.8${\pi}$ when using a NA 0.25 objective in experiment.
Interferometric speckle visibility spectroscopy (iSVS) for measuring decorrelation time and dynamics of moving samples with enhanced signal-to-noise ratio and relaxed reference requirements
Jun 30, 2023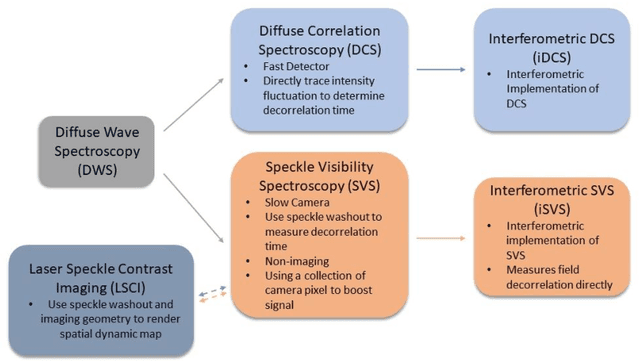
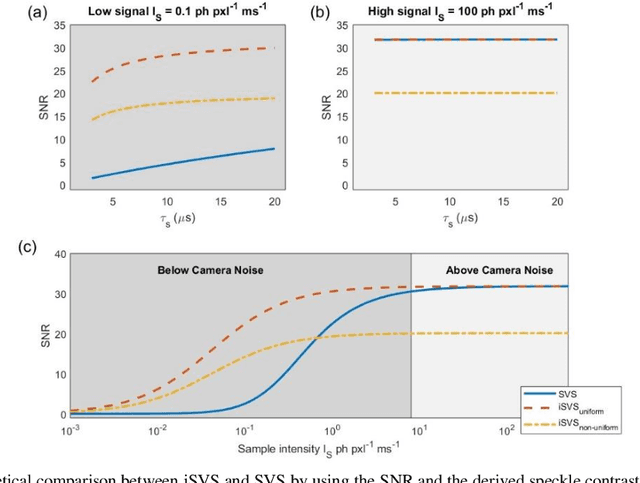
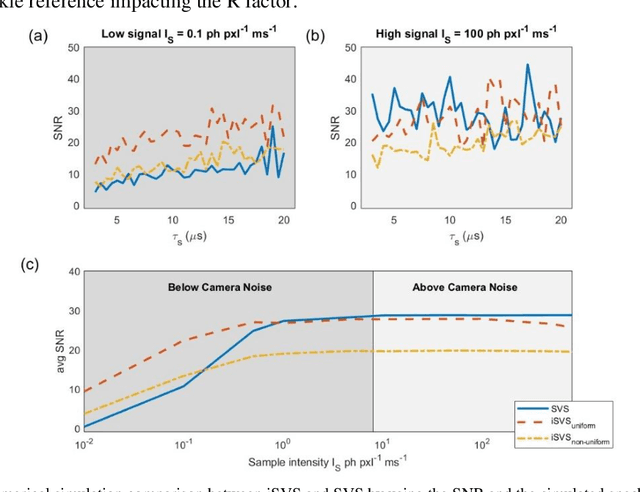
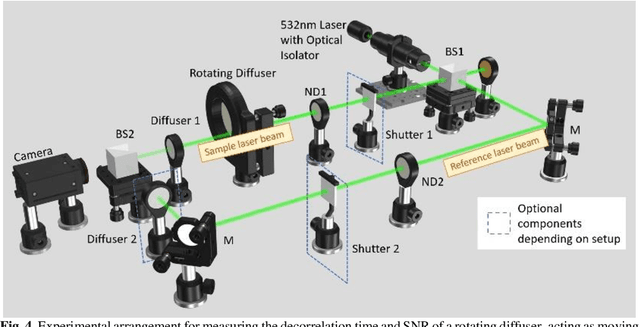
Abstract:Diffusing wave spectroscopy (DWS) is a group of techniques used to measure the dynamics of a scattering medium in a non-invasive manner. DWS methods rely on detecting the speckle light field from the moving scattering media and measuring the speckle decorrelation time to quantify the scattering mediums dynamics. For DWS, the signal-to-noise (SNR) is determined by the ratio between measured decorrelation time to the standard error of the measurement. This SNR is often low in certain applications because of high noise variances and low signal intensity, especially in biological applications with restricted exposure and emission levels. To address this photon-limited signal-to-noise ratio problem, we investigated, theoretically and experimentally, the SNR of an interferometric speckle visibility spectroscopy (iSVS) compared to more traditional DWS methods. We found that iSVS can provide excellent SNR performance through its ability to overcome camera noise. We also proved iSVS system has more relaxed constraints on the reference beam properties than most other interferometric systems. For an iSVS to function properly, we simply require the reference beam to exhibit local temporal stability, while incident angle, reference phase, and intensity uniformity do not need to be constrained. This flexibility can potentially enable more unconventional iSVS implementation schemes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge