Carole Frindel
CREATIS
Do Segmentation Models Understand Vascular Structure? A Blob-Based XAI Framework
Apr 11, 2025Abstract:Deep learning models have achieved impressive performance in medical image segmentation, yet their black-box nature limits clinical adoption. In vascular applications, trustworthy segmentation should rely on both local image cues and global anatomical structures, such as vessel connectivity or branching. However, the extent to which models leverage such global context remains unclear. We present a novel explainability pipeline for 3D vessel segmentation, combining gradient-based attribution with graph-guided point selection and a blob-based analysis of Saliency maps. Using vascular graphs extracted from ground truth, we define anatomically meaningful points of interest (POIs) and assess the contribution of input voxels via Saliency maps. These are analyzed at both global and local scales using a custom blob detector. Applied to IRCAD and Bullitt datasets, our analysis shows that model decisions are dominated by highly localized attribution blobs centered near POIs. Attribution features show little correlation with vessel-level properties such as thickness, tubularity, or connectivity -- suggesting limited use of global anatomical reasoning. Our results underline the importance of structured explainability tools and highlight the current limitations of segmentation models in capturing global vascular context.
A Robust Ensemble Algorithm for Ischemic Stroke Lesion Segmentation: Generalizability and Clinical Utility Beyond the ISLES Challenge
Apr 03, 2024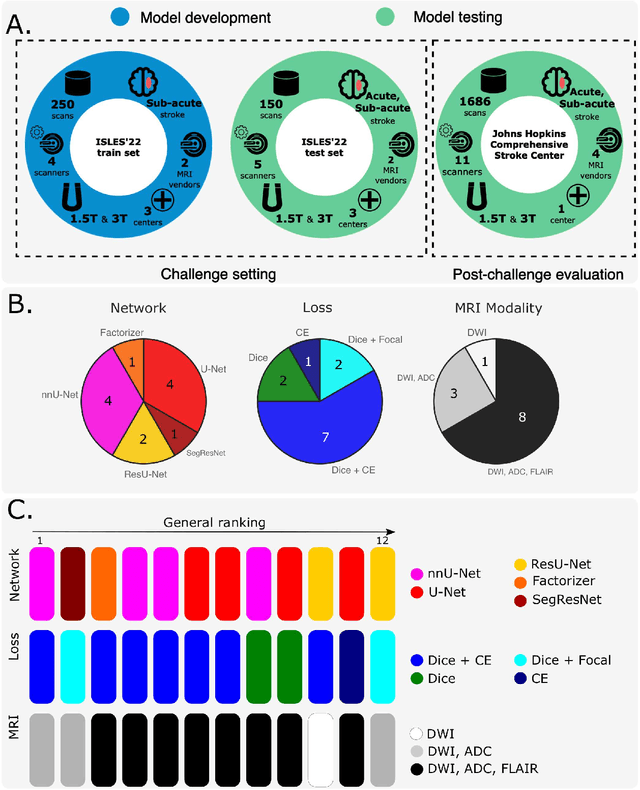
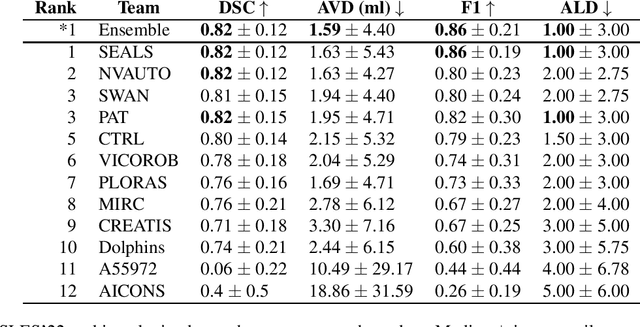
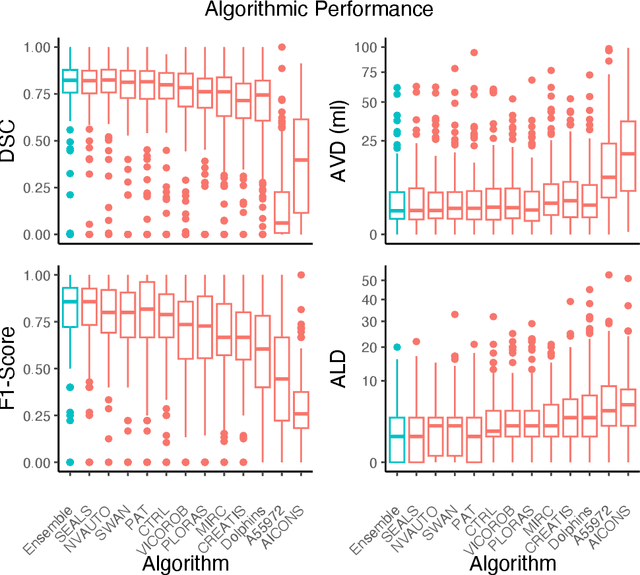
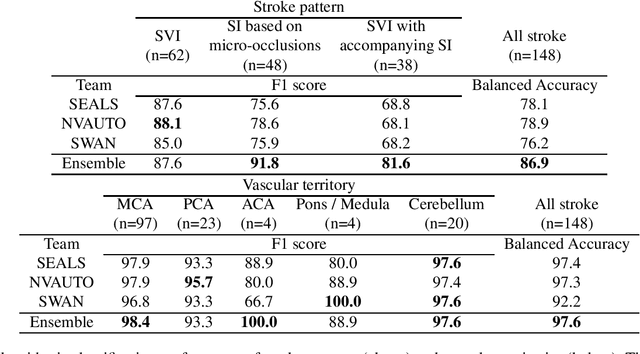
Abstract:Diffusion-weighted MRI (DWI) is essential for stroke diagnosis, treatment decisions, and prognosis. However, image and disease variability hinder the development of generalizable AI algorithms with clinical value. We address this gap by presenting a novel ensemble algorithm derived from the 2022 Ischemic Stroke Lesion Segmentation (ISLES) challenge. ISLES'22 provided 400 patient scans with ischemic stroke from various medical centers, facilitating the development of a wide range of cutting-edge segmentation algorithms by the research community. Through collaboration with leading teams, we combined top-performing algorithms into an ensemble model that overcomes the limitations of individual solutions. Our ensemble model achieved superior ischemic lesion detection and segmentation accuracy on our internal test set compared to individual algorithms. This accuracy generalized well across diverse image and disease variables. Furthermore, the model excelled in extracting clinical biomarkers. Notably, in a Turing-like test, neuroradiologists consistently preferred the algorithm's segmentations over manual expert efforts, highlighting increased comprehensiveness and precision. Validation using a real-world external dataset (N=1686) confirmed the model's generalizability. The algorithm's outputs also demonstrated strong correlations with clinical scores (admission NIHSS and 90-day mRS) on par with or exceeding expert-derived results, underlining its clinical relevance. This study offers two key findings. First, we present an ensemble algorithm (https://github.com/Tabrisrei/ISLES22_Ensemble) that detects and segments ischemic stroke lesions on DWI across diverse scenarios on par with expert (neuro)radiologists. Second, we show the potential for biomedical challenge outputs to extend beyond the challenge's initial objectives, demonstrating their real-world clinical applicability.
Deep vessel segmentation based on a new combination of vesselness filters
Feb 22, 2024Abstract:Vascular segmentation represents a crucial clinical task, yet its automation remains challenging. Because of the recent strides in deep learning, vesselness filters, which can significantly aid the learning process, have been overlooked. This study introduces an innovative filter fusion method crafted to amplify the effectiveness of vessel segmentation models. Our investigation seeks to establish the merits of a filter-based learning approach through a comparative analysis. Specifically, we contrast the performance of a U-Net model trained on CT images with an identical U-Net configuration trained on vesselness hyper-volumes using matching parameters. Our findings, based on two vascular datasets, highlight improved segmentations, especially for small vessels, when the model's learning is exposed to vessel-enhanced inputs.
A Novel Autoencoders-LSTM Model for Stroke Outcome Prediction using Multimodal MRI Data
Mar 16, 2023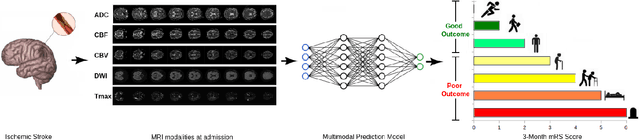
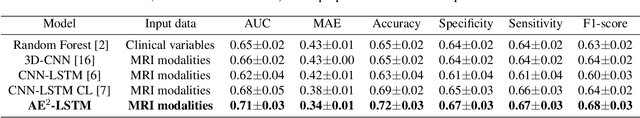
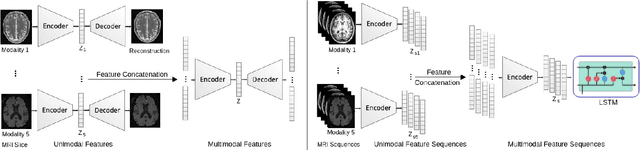
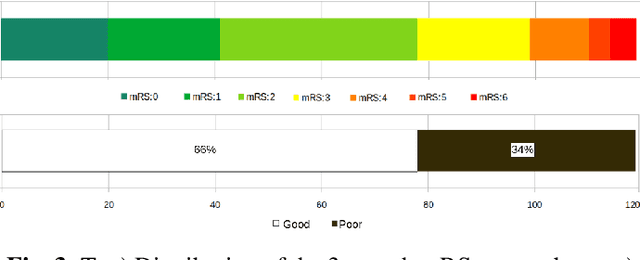
Abstract:Patient outcome prediction is critical in management of ischemic stroke. In this paper, a novel machine learning model is proposed for stroke outcome prediction using multimodal Magnetic Resonance Imaging (MRI). The proposed model consists of two serial levels of Autoencoders (AEs), where different AEs at level 1 are used for learning unimodal features from different MRI modalities and a AE at level 2 is used to combine the unimodal features into compressed multimodal features. The sequences of multimodal features of a given patient are then used by an LSTM network for predicting outcome score. The proposed AE2-LSTM model is proved to be an effective approach for better addressing the multimodality and volumetric nature of MRI data. Experimental results show that the proposed AE2-LSTM outperforms the existing state-of-the art models by achieving highest AUC=0.71 and lowest MAE=0.34.
CNN-LSTM Based Multimodal MRI and Clinical Data Fusion for Predicting Functional Outcome in Stroke Patients
May 11, 2022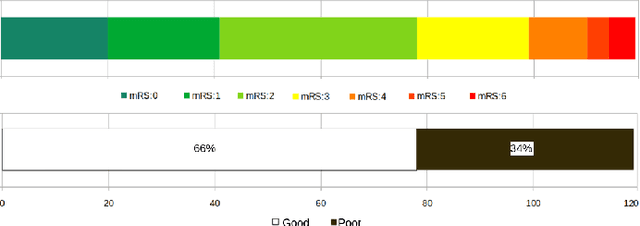
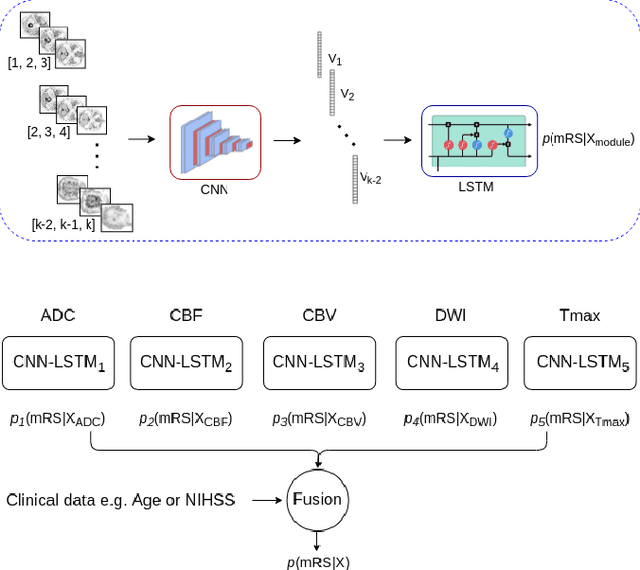
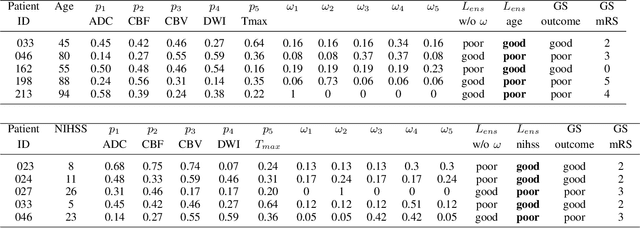
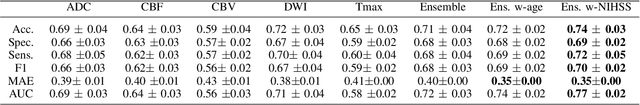
Abstract:Clinical outcome prediction plays an important role in stroke patient management. From a machine learning point-of-view, one of the main challenges is dealing with heterogeneous data at patient admission, i.e. the image data which are multidimensional and the clinical data which are scalars. In this paper, a multimodal convolutional neural network - long short-term memory (CNN-LSTM) based ensemble model is proposed. For each MR image module, a dedicated network provides preliminary prediction of the clinical outcome using the modified Rankin scale (mRS). The final mRS score is obtained by merging the preliminary probabilities of each module dedicated to a specific type of MR image weighted by the clinical metadata, here age or the National Institutes of Health Stroke Scale (NIHSS). The experimental results demonstrate that the proposed model surpasses the baselines and offers an original way to automatically encode the spatio-temporal context of MR images in a deep learning architecture. The highest AUC (0.77) was achieved for the proposed model with NIHSS.
Modeling and hexahedral meshing of arterial networks from centerlines
Jan 20, 2022



Abstract:Computational fluid dynamics (CFD) simulation provides valuable information on blood flow from the vascular geometry. However, it requires to extract accurate models of arteries from low resolution medical images, which remains challenging. Centerline-based representation is widely used to model large vascular networks with small vessels, as it enables manual editing and encodes the topological information. In this work, we propose an automatic method to generate an hexahedral mesh suitable for CFD directly from centerlines. The proposed method is an improvement of the state-of-the-art in terms of robustness, mesh quality and reproductibility. Both the modeling and meshing tasks are addressed. A new vessel model based on penalized splines is proposed to overcome the limitations inherent to the centerline representation, such as noise and sparsity. Bifurcations are reconstructed using a physiologically accurate parametric model that we extended to planar n-furcations. Finally, a volume mesh with structured, hexahedral and flow oriented cells is produced from the proposed vascular network model. The proposed method offers a better robustness and mesh quality than the state-of-the-art methods. As it combines both modeling and meshing techniques, it can be applied to edit the geometry and topology of vascular models effortlessly to study the impact on hemodynamics. We demonstrate the efficiency of our method by entirely meshing a dataset of 60 cerebral vascular networks. 92\% of the vessels and 83\% of the bifurcations where mesh without defects needing manual intervention, despite the challenging aspect of the input data. The source code will be released publicly.
Privacy Assessment of Federated Learning using Private Personalized Layers
Jun 15, 2021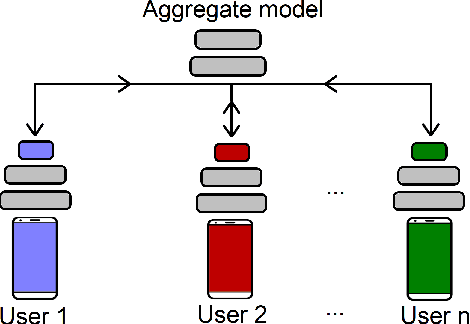
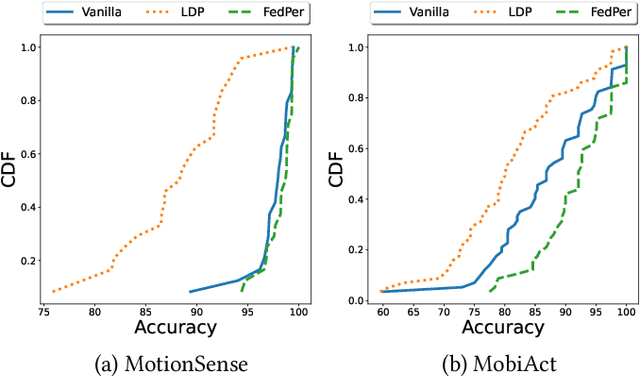
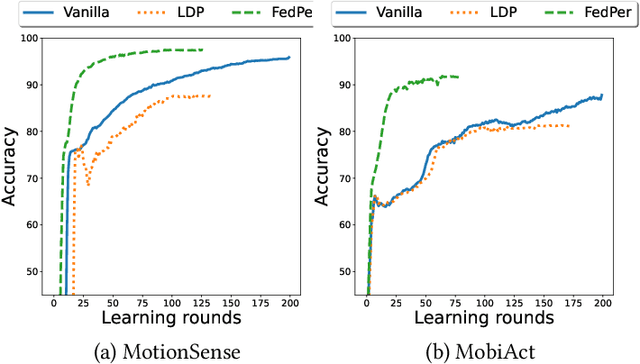
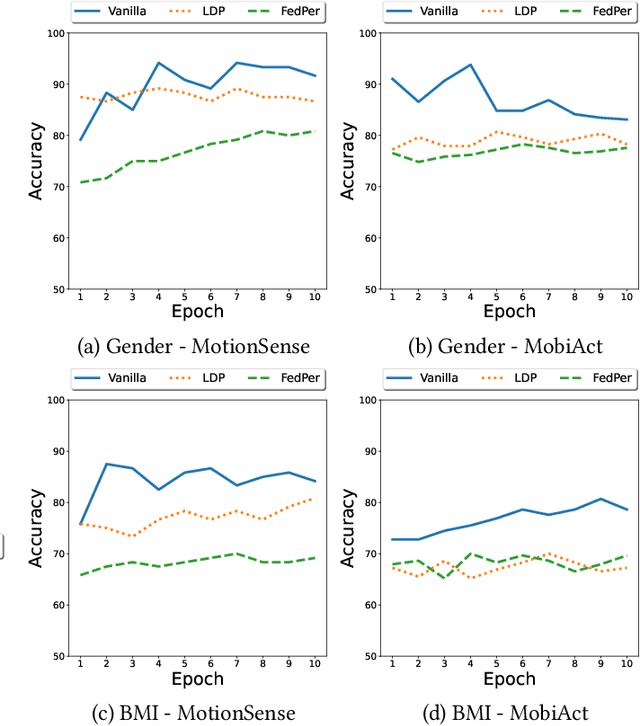
Abstract:Federated Learning (FL) is a collaborative scheme to train a learning model across multiple participants without sharing data. While FL is a clear step forward towards enforcing users' privacy, different inference attacks have been developed. In this paper, we quantify the utility and privacy trade-off of a FL scheme using private personalized layers. While this scheme has been proposed as local adaptation to improve the accuracy of the model through local personalization, it has also the advantage to minimize the information about the model exchanged with the server. However, the privacy of such a scheme has never been quantified. Our evaluations using motion sensor dataset show that personalized layers speed up the convergence of the model and slightly improve the accuracy for all users compared to a standard FL scheme while better preventing both attribute and membership inferences compared to a FL scheme using local differential privacy.
DYSAN: Dynamically sanitizing motion sensor data against sensitive inferences through adversarial networks
Mar 23, 2020



Abstract:With the widespread adoption of the quantified self movement, an increasing number of users rely on mobile applications to monitor their physical activity through their smartphones. Granting to applications a direct access to sensor data expose users to privacy risks. Indeed, usually these motion sensor data are transmitted to analytics applications hosted on the cloud leveraging machine learning models to provide feedback on their health to users. However, nothing prevents the service provider to infer private and sensitive information about a user such as health or demographic attributes.In this paper, we present DySan, a privacy-preserving framework to sanitize motion sensor data against unwanted sensitive inferences (i.e., improving privacy) while limiting the loss of accuracy on the physical activity monitoring (i.e., maintaining data utility). To ensure a good trade-off between utility and privacy, DySan leverages on the framework of Generative Adversarial Network (GAN) to sanitize the sensor data. More precisely, by learning in a competitive manner several networks, DySan is able to build models that sanitize motion data against inferences on a specified sensitive attribute (e.g., gender) while maintaining a high accuracy on activity recognition. In addition, DySan dynamically selects the sanitizing model which maximize the privacy according to the incoming data. Experiments conducted on real datasets demonstrate that DySan can drasticallylimit the gender inference to 47% while only reducing the accuracy of activity recognition by 3%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge