Berk Norman
Out-of-Distribution Detection & Applications With Ablated Learned Temperature Energy
Jan 22, 2024Abstract:As deep neural networks become adopted in high-stakes domains, it is crucial to be able to identify when inference inputs are Out-of-Distribution (OOD) so that users can be alerted of likely drops in performance and calibration despite high confidence. Among many others, existing methods use the following two scores to do so without training on any apriori OOD examples: a learned temperature and an energy score. In this paper we introduce Ablated Learned Temperature Energy (or "AbeT" for short), a method which combines these prior methods in novel ways with effective modifications. Due to these contributions, AbeT lowers the False Positive Rate at $95\%$ True Positive Rate (FPR@95) by $35.39\%$ in classification (averaged across all ID and OOD datasets measured) compared to state of the art without training networks in multiple stages or requiring hyperparameters or test-time backward passes. We additionally provide empirical insights as to how our model learns to distinguish between In-Distribution (ID) and OOD samples while only being explicitly trained on ID samples via exposure to misclassified ID examples at training time. Lastly, we show the efficacy of our method in identifying predicted bounding boxes and pixels corresponding to OOD objects in object detection and semantic segmentation, respectively - with an AUROC increase of $5.15\%$ in object detection and both a decrease in FPR@95 of $41.48\%$ and an increase in AUPRC of $34.20\%$ on average in semantic segmentation compared to previous state of the art.
ScarGAN: Chained Generative Adversarial Networks to Simulate Pathological Tissue on Cardiovascular MR Scans
Aug 14, 2018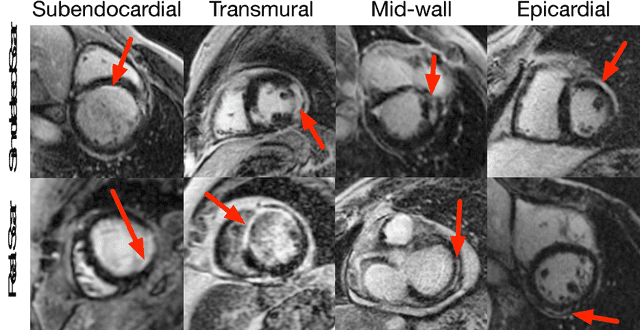
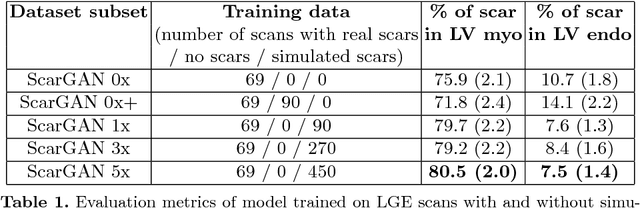
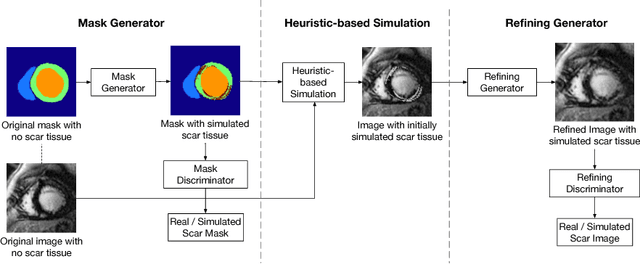
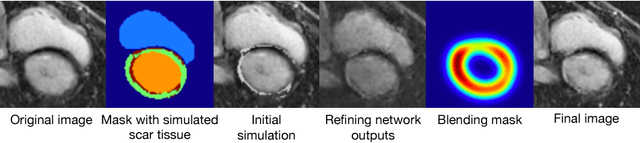
Abstract:Medical images with specific pathologies are scarce, but a large amount of data is usually required for a deep convolutional neural network (DCNN) to achieve good accuracy. We consider the problem of segmenting the left ventricular (LV) myocardium on late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR) scans of which only some of the scans have scar tissue. We propose ScarGAN to simulate scar tissue on healthy myocardium using chained generative adversarial networks (GAN). Our novel approach factorizes the simulation process into 3 steps: 1) a mask generator to simulate the shape of the scar tissue; 2) a domain-specific heuristic to produce the initial simulated scar tissue from the simulated shape; 3) a refining generator to add details to the simulated scar tissue. Unlike other approaches that generate samples from scratch, we simulate scar tissue on normal scans resulting in highly realistic samples. We show that experienced radiologists are unable to distinguish between real and simulated scar tissue. Training a U-Net with additional scans with scar tissue simulated by ScarGAN increases the percentage of scar pixels correctly included in LV myocardium prediction from 75.9% to 80.5%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge