Asim Ukaye
Introducing SDICE: An Index for Assessing Diversity of Synthetic Medical Datasets
Sep 28, 2024



Abstract:Advancements in generative modeling are pushing the state-of-the-art in synthetic medical image generation. These synthetic images can serve as an effective data augmentation method to aid the development of more accurate machine learning models for medical image analysis. While the fidelity of these synthetic images has progressively increased, the diversity of these images is an understudied phenomenon. In this work, we propose the SDICE index, which is based on the characterization of similarity distributions induced by a contrastive encoder. Given a synthetic dataset and a reference dataset of real images, the SDICE index measures the distance between the similarity score distributions of original and synthetic images, where the similarity scores are estimated using a pre-trained contrastive encoder. This distance is then normalized using an exponential function to provide a consistent metric that can be easily compared across domains. Experiments conducted on the MIMIC-chest X-ray and ImageNet datasets demonstrate the effectiveness of SDICE index in assessing synthetic medical dataset diversity.
UniLVSeg: Unified Left Ventricular Segmentation with Sparsely Annotated Echocardiogram Videos through Self-Supervised Temporal Masking and Weakly Supervised Training
Sep 30, 2023Abstract:Echocardiography has become an indispensable clinical imaging modality for general heart health assessment. From calculating biomarkers such as ejection fraction to the probability of a patient's heart failure, accurate segmentation of the heart and its structures allows doctors to plan and execute treatments with greater precision and accuracy. However, achieving accurate and robust left ventricle segmentation is time-consuming and challenging due to different reasons. This work introduces a novel approach for consistent left ventricular (LV) segmentation from sparsely annotated echocardiogram videos. We achieve this through (1) self-supervised learning (SSL) using temporal masking followed by (2) weakly supervised training. We investigate two different segmentation approaches: 3D segmentation and a novel 2D superimage (SI). We demonstrate how our proposed method outperforms the state-of-the-art solutions by achieving a 93.32% (95%CI 93.21-93.43%) dice score on a large-scale dataset (EchoNet-Dynamic) while being more efficient. To show the effectiveness of our approach, we provide extensive ablation studies, including pre-training settings and various deep learning backbones. Additionally, we discuss how our proposed methodology achieves high data utility by incorporating unlabeled frames in the training process. To help support the AI in medicine community, the complete solution with the source code will be made publicly available upon acceptance.
Leveraging the HW/SW Optimizations and Ecosystems that Drive the AI Revolution
Aug 04, 2022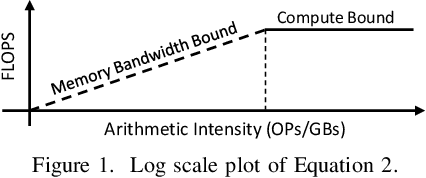
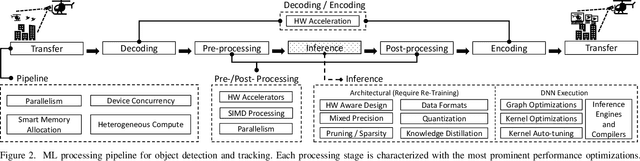
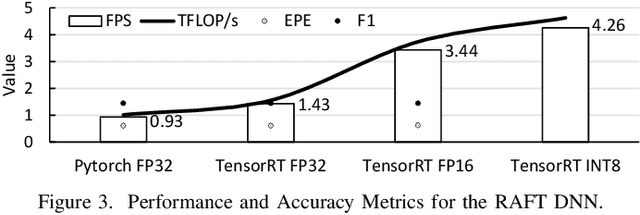
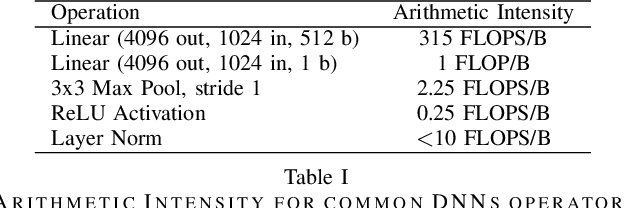
Abstract:This paper presents a state-of-the-art overview on how to architect, design, and optimize Deep Neural Networks (DNNs) such that performance is improved and accuracy is preserved. The paper covers a set of optimizations that span the entire Machine Learning processing pipeline. We introduce two types of optimizations. The first alters the DNN model and requires NN re-training, while the second does not. We focus on GPU optimizations, but we believe the presented techniques can be used with other AI inference platforms. To demonstrate the DNN model optimizations, we improve one of the most advanced deep network architectures for optical flow, RAFT arXiv:2003.12039, on a popular edge AI inference platform (Nvidia Jetson AGX Xavier).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge