Alex Lu
Domain adaptation using optimal transport for invariant learning using histopathology datasets
Mar 03, 2023Abstract:Histopathology is critical for the diagnosis of many diseases, including cancer. These protocols typically require pathologists to manually evaluate slides under a microscope, which is time-consuming and subjective, leading to interest in machine learning to automate analysis. However, computational techniques are limited by batch effects, where technical factors like differences in preparation protocol or scanners can alter the appearance of slides, causing models trained on one institution to fail when generalizing to others. Here, we propose a domain adaptation method that improves the generalization of histopathological models to data from unseen institutions, without the need for labels or retraining in these new settings. Our approach introduces an optimal transport (OT) loss, that extends adversarial methods that penalize models if images from different institutions can be distinguished in their representation space. Unlike previous methods, which operate on single samples, our loss accounts for distributional differences between batches of images. We show that on the Camelyon17 dataset, while both methods can adapt to global differences in color distribution, only our OT loss can reliably classify a cancer phenotype unseen during training. Together, our results suggest that OT improves generalization on rare but critical phenotypes that may only make up a small fraction of the total tiles and variation in a slide.
Improved Conditional Flow Models for Molecule to Image Synthesis
Jun 15, 2020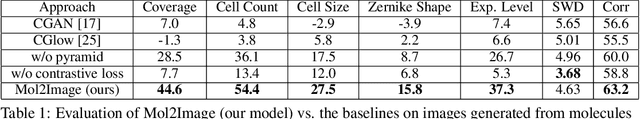
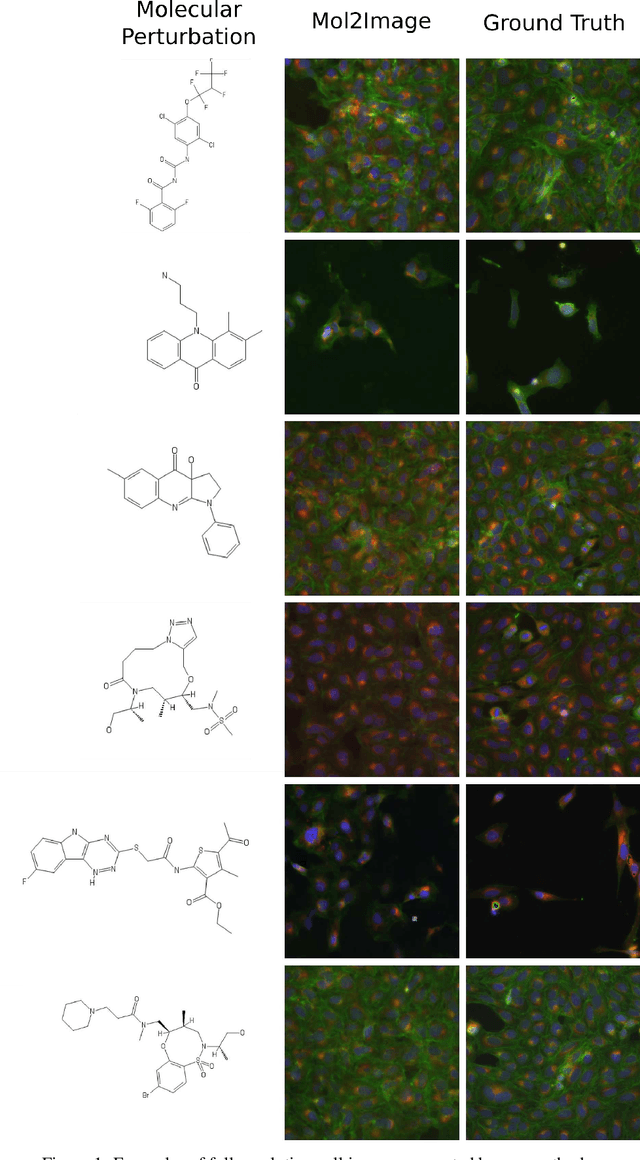
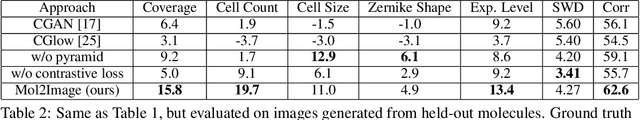
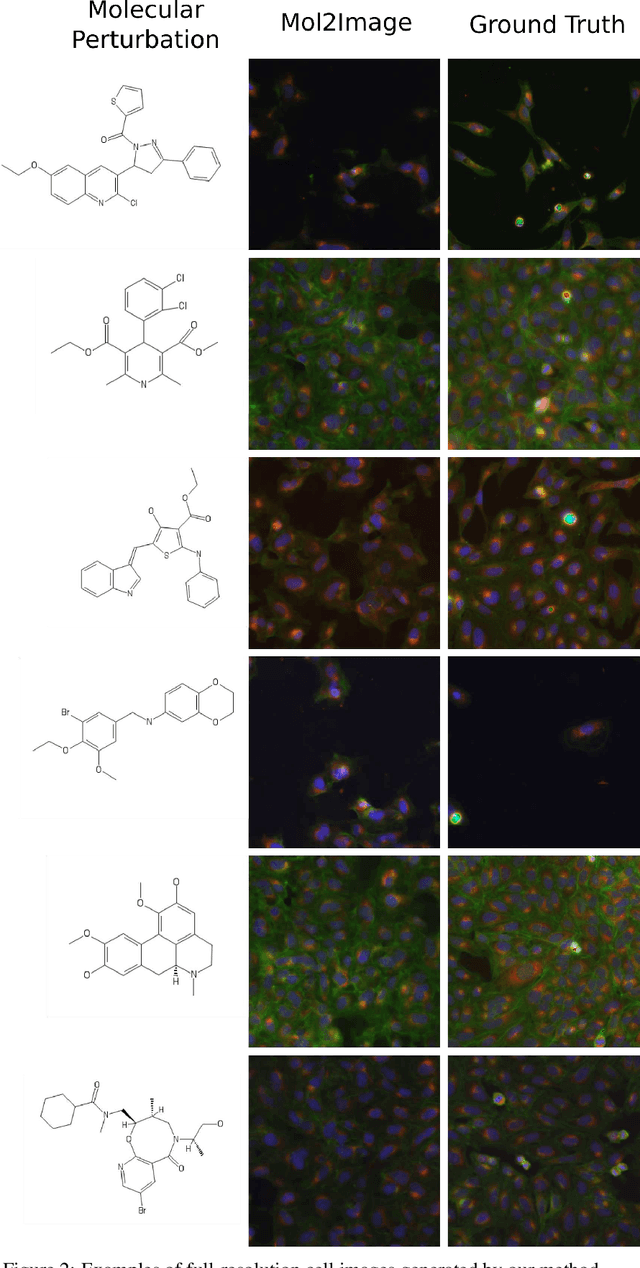
Abstract:In this paper, we aim to synthesize cell microscopy images under different molecular interventions, motivated by practical applications to drug development. Building on the recent success of graph neural networks for learning molecular embeddings and flow-based models for image generation, we propose Mol2Image: a flow-based generative model for molecule to cell image synthesis. To generate cell features at different resolutions and scale to high-resolution images, we develop a novel multi-scale flow architecture based on a Haar wavelet image pyramid. To maximize the mutual information between the generated images and the molecular interventions, we devise a training strategy based on contrastive learning. To evaluate our model, we propose a new set of metrics for biological image generation that are robust, interpretable, and relevant to practitioners. We show quantitatively that our method learns a meaningful embedding of the molecular intervention, which is translated into an image representation reflecting the biological effects of the intervention.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge