Akshit Srivastava
INSITE: labelling medical images using submodular functions and semi-supervised data programming
Feb 11, 2024
Abstract:The necessity of large amounts of labeled data to train deep models, especially in medical imaging creates an implementation bottleneck in resource-constrained settings. In Insite (labelINg medical imageS usIng submodular funcTions and sEmi-supervised data programming) we apply informed subset selection to identify a small number of most representative or diverse images from a huge pool of unlabelled data subsequently annotated by a domain expert. The newly annotated images are then used as exemplars to develop several data programming-driven labeling functions. These labelling functions output a predicted-label and a similarity score when given an unlabelled image as an input. A consensus is brought amongst the outputs of these labeling functions by using a label aggregator function to assign the final predicted label to each unlabelled data point. We demonstrate that informed subset selection followed by semi-supervised data programming methods using these images as exemplars perform better than other state-of-the-art semi-supervised methods. Further, for the first time we demonstrate that this can be achieved through a small set of images used as exemplars.
DIAGNOSE: Avoiding Out-of-distribution Data using Submodular Information Measures
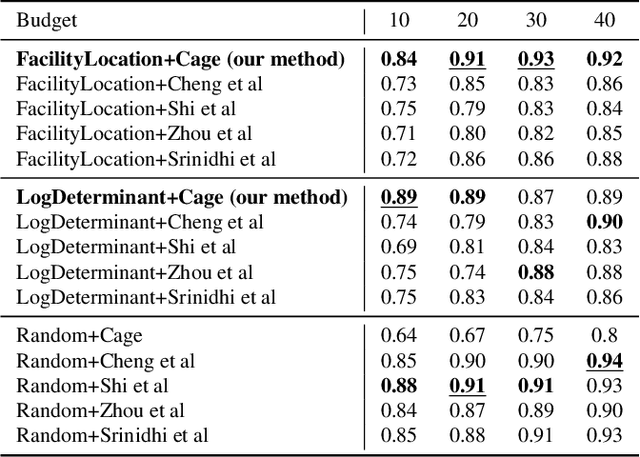
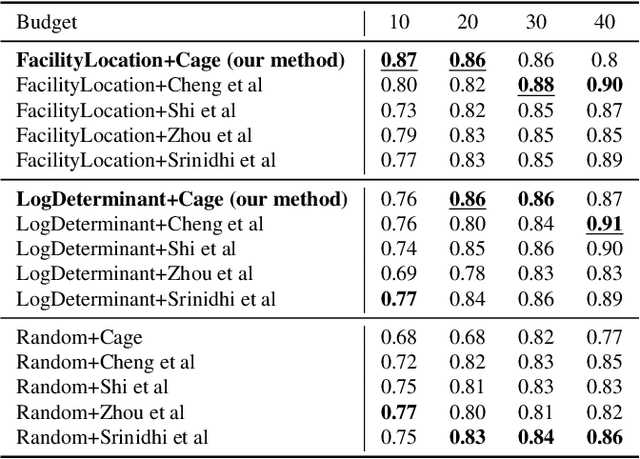
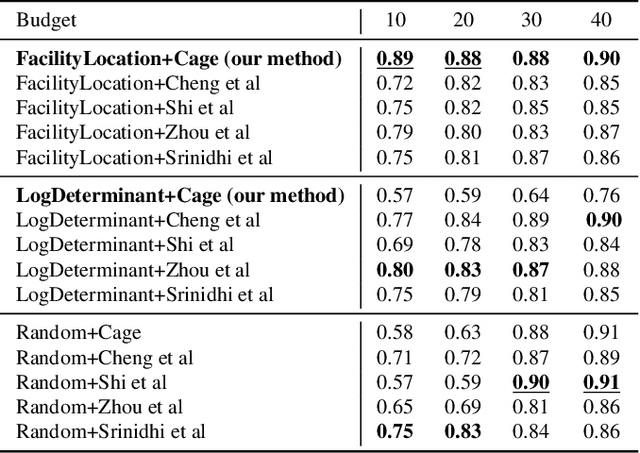
Oct 04, 2022Abstract:Avoiding out-of-distribution (OOD) data is critical for training supervised machine learning models in the medical imaging domain. Furthermore, obtaining labeled medical data is difficult and expensive since it requires expert annotators like doctors, radiologists, etc. Active learning (AL) is a well-known method to mitigate labeling costs by selecting the most diverse or uncertain samples. However, current AL methods do not work well in the medical imaging domain with OOD data. We propose Diagnose (avoiDing out-of-dIstribution dAta usinG submodular iNfOrmation meaSurEs), a novel active learning framework that can jointly model similarity and dissimilarity, which is crucial in mining in-distribution data and avoiding OOD data at the same time. Particularly, we use a small number of data points as exemplars that represent a query set of in-distribution data points and a private set of OOD data points. We illustrate the generalizability of our framework by evaluating it on a wide variety of real-world OOD scenarios. Our experiments verify the superiority of Diagnose over the state-of-the-art AL methods across multiple domains of medical imaging.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge