Yide Zhang
4KAgent: Agentic Any Image to 4K Super-Resolution
Jul 09, 2025Abstract:We present 4KAgent, a unified agentic super-resolution generalist system designed to universally upscale any image to 4K resolution (and even higher, if applied iteratively). Our system can transform images from extremely low resolutions with severe degradations, for example, highly distorted inputs at 256x256, into crystal-clear, photorealistic 4K outputs. 4KAgent comprises three core components: (1) Profiling, a module that customizes the 4KAgent pipeline based on bespoke use cases; (2) A Perception Agent, which leverages vision-language models alongside image quality assessment experts to analyze the input image and make a tailored restoration plan; and (3) A Restoration Agent, which executes the plan, following a recursive execution-reflection paradigm, guided by a quality-driven mixture-of-expert policy to select the optimal output for each step. Additionally, 4KAgent embeds a specialized face restoration pipeline, significantly enhancing facial details in portrait and selfie photos. We rigorously evaluate our 4KAgent across 11 distinct task categories encompassing a total of 26 diverse benchmarks, setting new state-of-the-art on a broad spectrum of imaging domains. Our evaluations cover natural images, portrait photos, AI-generated content, satellite imagery, fluorescence microscopy, and medical imaging like fundoscopy, ultrasound, and X-ray, demonstrating superior performance in terms of both perceptual (e.g., NIQE, MUSIQ) and fidelity (e.g., PSNR) metrics. By establishing a novel agentic paradigm for low-level vision tasks, we aim to catalyze broader interest and innovation within vision-centric autonomous agents across diverse research communities. We will release all the code, models, and results at: https://4kagent.github.io.
Single-shot 3D photoacoustic computed tomography with a densely packed array for transcranial functional imaging
Jun 26, 2023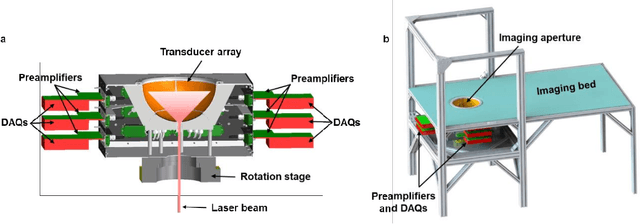
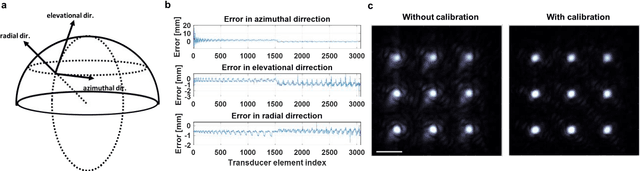
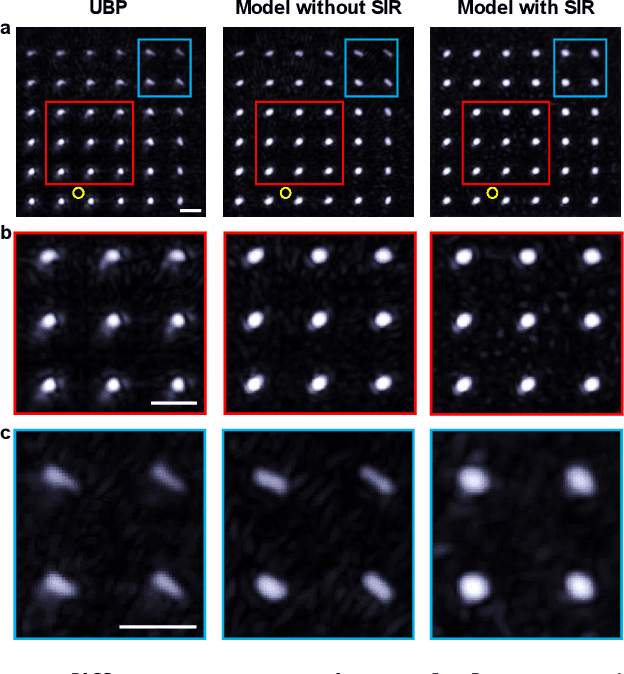
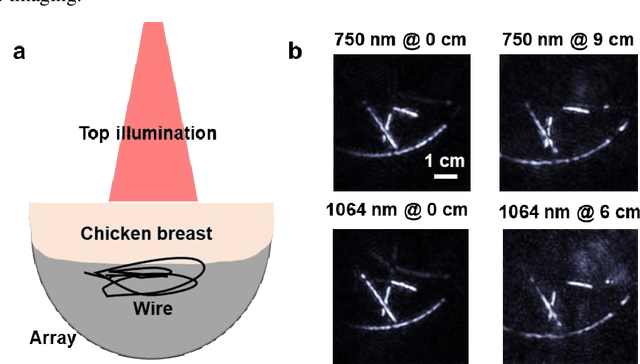
Abstract:Photoacoustic computed tomography (PACT) is emerging as a new technique for functional brain imaging, primarily due to its capabilities in label-free hemodynamic imaging. Despite its potential, the transcranial application of PACT has encountered hurdles, such as acoustic attenuations and distortions by the skull and limited light penetration through the skull. To overcome these challenges, we have engineered a PACT system that features a densely packed hemispherical ultrasonic transducer array with 3072 channels, operating at a central frequency of 1 MHz. This system allows for single-shot 3D imaging at a rate equal to the laser repetition rate, such as 20 Hz. We have achieved a single-shot light penetration depth of approximately 9 cm in chicken breast tissue utilizing a 750 nm laser (withstanding 3295-fold light attenuation and still retaining an SNR of 74) and successfully performed transcranial imaging through an ex vivo human skull using a 1064 nm laser. Moreover, we have proven the capacity of our system to perform single-shot 3D PACT imaging in both tissue phantoms and human subjects. These results suggest that our PACT system is poised to unlock potential for real-time, in vivo transcranial functional imaging in humans.
Convolutional Neural Network Denoising in Fluorescence Lifetime Imaging Microscopy (FLIM)
Mar 07, 2021



Abstract:Fluorescence lifetime imaging microscopy (FLIM) systems are limited by their slow processing speed, low signal-to-noise ratio (SNR), and expensive and challenging hardware setups. In this work, we demonstrate applying a denoising convolutional network to improve FLIM SNR. The network will be integrated with an instant FLIM system with fast data acquisition based on analog signal processing, high SNR using high-efficiency pulse-modulation, and cost-effective implementation utilizing off-the-shelf radio-frequency components. Our instant FLIM system simultaneously provides the intensity, lifetime, and phasor plots \textit{in vivo} and \textit{ex vivo}. By integrating image denoising using the trained deep learning model on the FLIM data, provide accurate FLIM phasor measurements are obtained. The enhanced phasor is then passed through the K-means clustering segmentation method, an unbiased and unsupervised machine learning technique to separate different fluorophores accurately. Our experimental \textit{in vivo} mouse kidney results indicate that introducing the deep learning image denoising model before the segmentation effectively removes the noise in the phasor compared to existing methods and provides clearer segments. Hence, the proposed deep learning-based workflow provides fast and accurate automatic segmentation of fluorescence images using instant FLIM. The denoising operation is effective for the segmentation if the FLIM measurements are noisy. The clustering can effectively enhance the detection of biological structures of interest in biomedical imaging applications.
Deep learning-based super-resolution fluorescence microscopy on small datasets
Mar 07, 2021



Abstract:Fluorescence microscopy has enabled a dramatic development in modern biology by visualizing biological organisms with micrometer scale resolution. However, due to the diffraction limit, sub-micron/nanometer features are difficult to resolve. While various super-resolution techniques are developed to achieve nanometer-scale resolution, they often either require expensive optical setup or specialized fluorophores. In recent years, deep learning has shown the potentials to reduce the technical barrier and obtain super-resolution from diffraction-limited images. For accurate results, conventional deep learning techniques require thousands of images as a training dataset. Obtaining large datasets from biological samples is not often feasible due to the photobleaching of fluorophores, phototoxicity, and dynamic processes occurring within the organism. Therefore, achieving deep learning-based super-resolution using small datasets is challenging. We address this limitation with a new convolutional neural network-based approach that is successfully trained with small datasets and achieves super-resolution images. We captured 750 images in total from 15 different field-of-views as the training dataset to demonstrate the technique. In each FOV, a single target image is generated using the super-resolution radial fluctuation method. As expected, this small dataset failed to produce a usable model using traditional super-resolution architecture. However, using the new approach, a network can be trained to achieve super-resolution images from this small dataset. This deep learning model can be applied to other biomedical imaging modalities such as MRI and X-ray imaging, where obtaining large training datasets is challenging.
Machine learning for faster and smarter fluorescence lifetime imaging microscopy
Aug 05, 2020



Abstract:Fluorescence lifetime imaging microscopy (FLIM) is a powerful technique in biomedical research that uses the fluorophore decay rate to provide additional contrast in fluorescence microscopy. However, at present, the calculation, analysis, and interpretation of FLIM is a complex, slow, and computationally expensive process. Machine learning (ML) techniques are well suited to extract and interpret measurements from multi-dimensional FLIM data sets with substantial improvement in speed over conventional methods. In this topical review, we first discuss the basics of FILM and ML. Second, we provide a summary of lifetime extraction strategies using ML and its applications in classifying and segmenting FILM images with higher accuracy compared to conventional methods. Finally, we discuss two potential directions to improve FLIM with ML with proof of concept demonstrations.
A Poisson-Gaussian Denoising Dataset with Real Fluorescence Microscopy Images
Dec 26, 2018



Abstract:Fluorescence microscopy has enabled a dramatic development in modern biology. Due to its inherently weak signal, fluorescence microscopy is not only much noisier than photography, but also presented with Poisson-Gaussian noise where Poisson noise, or shot noise, is the dominating noise source, instead of Gaussian noise that dominates in photography. To get clean fluorescence microscopy images, it is highly desirable to have effective denoising algorithms and datasets that are specifically designed to denoise fluorescence microscopy images. While such algorithms exist, there are no such datasets available. In this paper, we fill this gap by constructing a dataset - the Fluorescence Microscopy Denoising (FMD) dataset - that is dedicated to Poisson-Gaussian denoising. The dataset consists 12,000 real fluorescence microscopy images obtained with commercial confocal, two-photon, and wide-field microscopes and representative biological samples such as cells, zebrafish, and mouse brain tissues. We use imaging averaging to effectively obtain ground truth images and 60,000 noisy images with different noise levels. We use this dataset to benchmark 10 representative denoising algorithms and find that deep learning methods have the best performance. To our knowledge, this is the first microscopy image dataset for Poisson-Gaussian denoising purposes and it could be an important tool for high-quality, real-time denoising applications in biomedical research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge