Vignesh Subbian
Failure Modes of Time Series Interpretability Algorithms for Critical Care Applications and Potential Solutions
Jun 23, 2025Abstract:Interpretability plays a vital role in aligning and deploying deep learning models in critical care, especially in constantly evolving conditions that influence patient survival. However, common interpretability algorithms face unique challenges when applied to dynamic prediction tasks, where patient trajectories evolve over time. Gradient, Occlusion, and Permutation-based methods often struggle with time-varying target dependency and temporal smoothness. This work systematically analyzes these failure modes and supports learnable mask-based interpretability frameworks as alternatives, which can incorporate temporal continuity and label consistency constraints to learn feature importance over time. Here, we propose that learnable mask-based approaches for dynamic timeseries prediction problems provide more reliable and consistent interpretations for applications in critical care and similar domains.
PHEONA: An Evaluation Framework for Large Language Model-based Approaches to Computational Phenotyping
Mar 25, 2025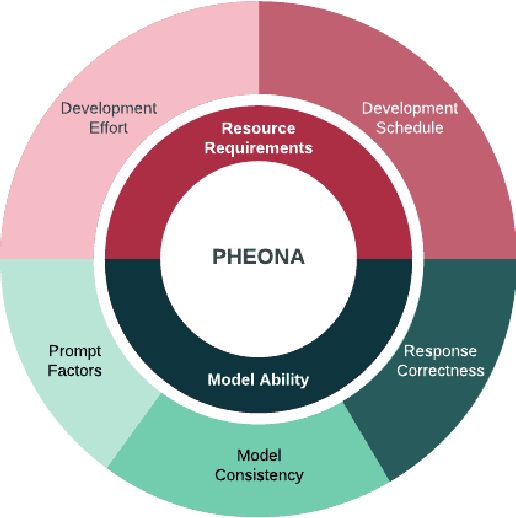
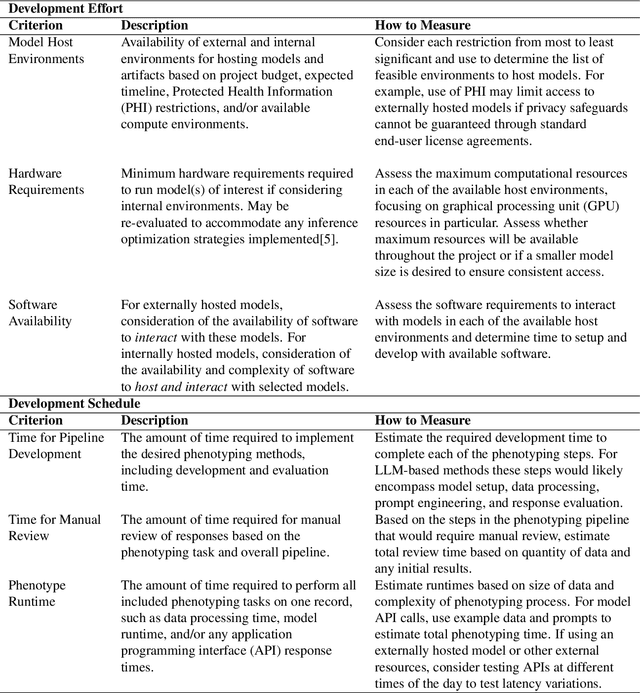
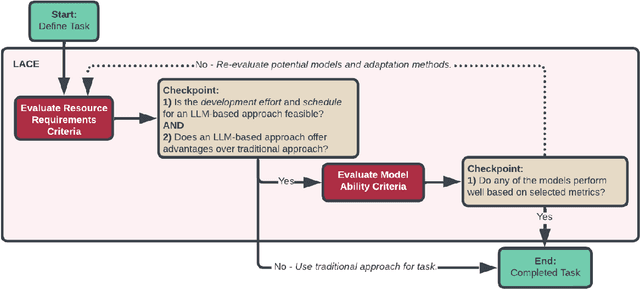
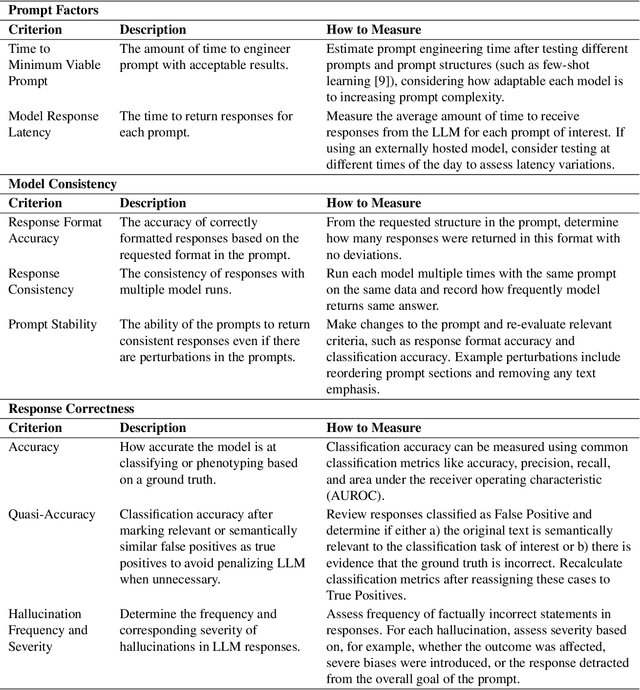
Abstract:Computational phenotyping is essential for biomedical research but often requires significant time and resources, especially since traditional methods typically involve extensive manual data review. While machine learning and natural language processing advancements have helped, further improvements are needed. Few studies have explored using Large Language Models (LLMs) for these tasks despite known advantages of LLMs for text-based tasks. To facilitate further research in this area, we developed an evaluation framework, Evaluation of PHEnotyping for Observational Health Data (PHEONA), that outlines context-specific considerations. We applied and demonstrated PHEONA on concept classification, a specific task within a broader phenotyping process for Acute Respiratory Failure (ARF) respiratory support therapies. From the sample concepts tested, we achieved high classification accuracy, suggesting the potential for LLM-based methods to improve computational phenotyping processes.
Discovery of Generalizable TBI Phenotypes Using Multivariate Time-Series Clustering
Jan 15, 2024


Abstract:Traumatic Brain Injury (TBI) presents a broad spectrum of clinical presentations and outcomes due to its inherent heterogeneity, leading to diverse recovery trajectories and varied therapeutic responses. While many studies have delved into TBI phenotyping for distinct patient populations, identifying TBI phenotypes that consistently generalize across various settings and populations remains a critical research gap. Our research addresses this by employing multivariate time-series clustering to unveil TBI's dynamic intricates. Utilizing a self-supervised learning-based approach to clustering multivariate time-Series data with missing values (SLAC-Time), we analyzed both the research-centric TRACK-TBI and the real-world MIMIC-IV datasets. Remarkably, the optimal hyperparameters of SLAC-Time and the ideal number of clusters remained consistent across these datasets, underscoring SLAC-Time's stability across heterogeneous datasets. Our analysis revealed three generalizable TBI phenotypes ({\alpha}, \b{eta}, and {\gamma}), each exhibiting distinct non-temporal features during emergency department visits, and temporal feature profiles throughout ICU stays. Specifically, phenotype {\alpha} represents mild TBI with a remarkably consistent clinical presentation. In contrast, phenotype \b{eta} signifies severe TBI with diverse clinical manifestations, and phenotype {\gamma} represents a moderate TBI profile in terms of severity and clinical diversity. Age is a significant determinant of TBI outcomes, with older cohorts recording higher mortality rates. Importantly, while certain features varied by age, the core characteristics of TBI manifestations tied to each phenotype remain consistent across diverse populations.
Identifying TBI Physiological States by Clustering of Multivariate Clinical Time-Series
Mar 30, 2023



Abstract:Determining clinically relevant physiological states from multivariate time series data with missing values is essential for providing appropriate treatment for acute conditions such as Traumatic Brain Injury (TBI), respiratory failure, and heart failure. Utilizing non-temporal clustering or data imputation and aggregation techniques may lead to loss of valuable information and biased analyses. In our study, we apply the SLAC-Time algorithm, an innovative self-supervision-based approach that maintains data integrity by avoiding imputation or aggregation, offering a more useful representation of acute patient states. By using SLAC-Time to cluster data in a large research dataset, we identified three distinct TBI physiological states and their specific feature profiles. We employed various clustering evaluation metrics and incorporated input from a clinical domain expert to validate and interpret the identified physiological states. Further, we discovered how specific clinical events and interventions can influence patient states and state transitions.
A Self-Supervised Learning-based Approach to Clustering Multivariate Time-Series Data with Missing Values : An Application to Traumatic Brain Injury Phenotyping
Feb 27, 2023



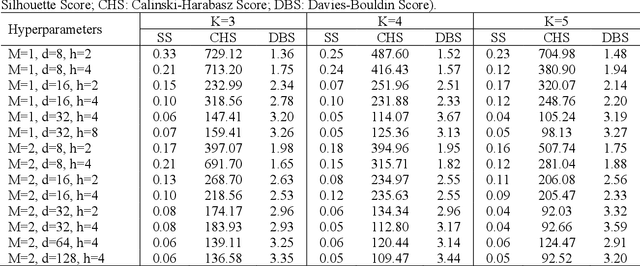
Abstract:Self-supervised learning approaches provide a promising direction for clustering multivariate time-series data. However, real-world time-series data often include missing values, and the existing approaches require imputing missing values before clustering, which may cause extensive computations and noise and result in invalid interpretations. To address these challenges, we present a Self-supervised Learning-based Approach to Clustering multivariate Time-series data with missing values (SLAC-Time). SLAC-Time is a Transformer-based clustering method that uses time-series forecasting as a proxy task for leveraging unlabeled data and learning more robust time-series representations. This method jointly learns the neural network parameters and the cluster assignments of the learned representations. It iteratively clusters the learned representations with the K-means method and then utilizes the subsequent cluster assignments as pseudo-labels to update the model parameters. To evaluate our proposed approach, we applied it to clustering and phenotyping Traumatic Brain Injury (TBI) patients in the TRACK-TBI dataset. Our experiments demonstrate that SLAC-Time outperforms the baseline K-means clustering algorithm in terms of silhouette coefficient, Calinski Harabasz index, Dunn index, and Davies Bouldin index. We identified three TBI phenotypes that are distinct from one another in terms of clinically significant variables as well as clinical outcomes, including the Extended Glasgow Outcome Scale (GOSE) score, Intensive Care Unit (ICU) length of stay, and mortality rate. The experiments show that the TBI phenotypes identified by SLAC-Time can be potentially used for developing targeted clinical trials and therapeutic strategies.
WindowSHAP: An Efficient Framework for Explaining Time-series Classifiers based on Shapley Values
Nov 11, 2022Abstract:Unpacking and comprehending how deep learning algorithms make decisions has been a persistent challenge for researchers and end-users. Explaining time-series predictive models is useful for clinical applications with high stakes to understand the behavior of prediction models. However, existing approaches to explain such models are frequently unique to architectures and data where the features do not have a time-varying component. In this paper, we introduce WindowSHAP, a model-agnostic framework for explaining time-series classifiers using Shapley values. We intend for WindowSHAP to mitigate the computational complexity of calculating Shapley values for long time-series data as well as improve the quality of explanations. WindowSHAP is based on partitioning a sequence into time windows. Under this framework, we present three distinct algorithms of Stationary, Sliding and Dynamic WindowSHAP, each evaluated against baseline approaches, KernelSHAP and TimeSHAP, using perturbation and sequence analyses metrics. We applied our framework to clinical time-series data from both a specialized clinical domain (Traumatic Brain Injury - TBI) as well as a broad clinical domain (critical care medicine). The experimental results demonstrate that, based on the two quantitative metrics, our framework is superior at explaining clinical time-series classifiers, while also reducing the complexity of computations. We show that for time-series data with 120 time steps (hours), merging 10 adjacent time points can reduce the CPU time of WindowSHAP by 80% compared to KernelSHAP. We also show that our Dynamic WindowSHAP algorithm focuses more on the most important time steps and provides more understandable explanations. As a result, WindowSHAP not only accelerates the calculation of Shapley values for time-series data, but also delivers more understandable explanations with higher quality.
An Empirical Comparison of Explainable Artificial Intelligence Methods for Clinical Data: A Case Study on Traumatic Brain Injury
Aug 13, 2022



Abstract:A longstanding challenge surrounding deep learning algorithms is unpacking and understanding how they make their decisions. Explainable Artificial Intelligence (XAI) offers methods to provide explanations of internal functions of algorithms and reasons behind their decisions in ways that are interpretable and understandable to human users. . Numerous XAI approaches have been developed thus far, and a comparative analysis of these strategies seems necessary to discern their relevance to clinical prediction models. To this end, we first implemented two prediction models for short- and long-term outcomes of traumatic brain injury (TBI) utilizing structured tabular as well as time-series physiologic data, respectively. Six different interpretation techniques were used to describe both prediction models at the local and global levels. We then performed a critical analysis of merits and drawbacks of each strategy, highlighting the implications for researchers who are interested in applying these methodologies. The implemented methods were compared to one another in terms of several XAI characteristics such as understandability, fidelity, and stability. Our findings show that SHAP is the most stable with the highest fidelity but falls short of understandability. Anchors, on the other hand, is the most understandable approach, but it is only applicable to tabular data and not time series data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge