Víctor M. Batlle
EndoMetric: Near-light metric scale monocular SLAM
Oct 19, 2024

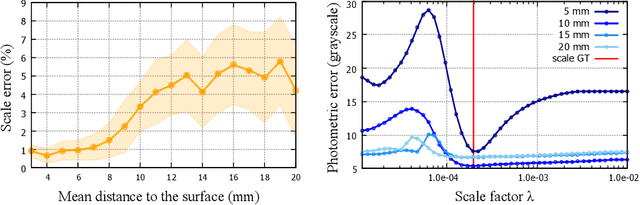
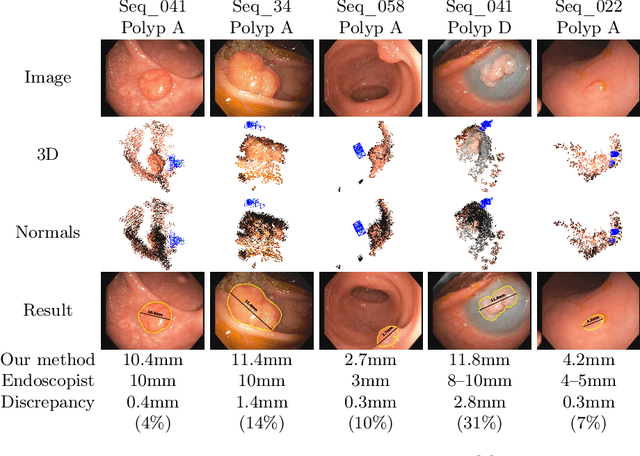
Abstract:Geometric reconstruction and SLAM with endoscopic images have seen significant advancements in recent years. In most medical specialties, the endoscopes used are monocular, and the algorithms applied are typically extensions of those designed for external environments, resulting in 3D reconstructions up to an unknown scale factor. In this paper, we take advantage of the fact that standard endoscopes are equipped with near-light sources positioned at a small but non-zero baseline from the camera. By leveraging the inverse-square law of light decay, we enable, for the first time, monocular reconstructions with accurate metric scale. This paves the way to transform any endoscope into a metric device, which is essential for practical applications such as measuring polyps, stenosis, or the extent of tissue affected by disease.
LightNeuS: Neural Surface Reconstruction in Endoscopy using Illumination Decline
Sep 06, 2023Abstract:We propose a new approach to 3D reconstruction from sequences of images acquired by monocular endoscopes. It is based on two key insights. First, endoluminal cavities are watertight, a property naturally enforced by modeling them in terms of a signed distance function. Second, the scene illumination is variable. It comes from the endoscope's light sources and decays with the inverse of the squared distance to the surface. To exploit these insights, we build on NeuS, a neural implicit surface reconstruction technique with an outstanding capability to learn appearance and a SDF surface model from multiple views, but currently limited to scenes with static illumination. To remove this limitation and exploit the relation between pixel brightness and depth, we modify the NeuS architecture to explicitly account for it and introduce a calibrated photometric model of the endoscope's camera and light source. Our method is the first one to produce watertight reconstructions of whole colon sections. We demonstrate excellent accuracy on phantom imagery. Remarkably, the watertight prior combined with illumination decline, allows to complete the reconstruction of unseen portions of the surface with acceptable accuracy, paving the way to automatic quality assessment of cancer screening explorations, measuring the global percentage of observed mucosa.
LightDepth: Single-View Depth Self-Supervision from Illumination Decline
Aug 21, 2023Abstract:Single-view depth estimation can be remarkably effective if there is enough ground-truth depth data for supervised training. However, there are scenarios, especially in medicine in the case of endoscopies, where such data cannot be obtained. In such cases, multi-view self-supervision and synthetic-to-real transfer serve as alternative approaches, however, with a considerable performance reduction in comparison to supervised case. Instead, we propose a single-view self-supervised method that achieves a performance similar to the supervised case. In some medical devices, such as endoscopes, the camera and light sources are co-located at a small distance from the target surfaces. Thus, we can exploit that, for any given albedo and surface orientation, pixel brightness is inversely proportional to the square of the distance to the surface, providing a strong single-view self-supervisory signal. In our experiments, our self-supervised models deliver accuracies comparable to those of fully supervised ones, while being applicable without depth ground-truth data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge